Class 9 SELINA Solutions Chemistry Chapter 6 - Study of the First Element - Hydrogen
Study of the First Element - Hydrogen Exercise Ex. 6(A)
Solution 1
Hydrogen is the first element in the periodic table. Its atomic number is 1, and it has only one electron in its valence shell. So, it belongs to the first group and the first period of the periodic table.
Solution 2
Hydrogen shows dual nature because it resembles the alkali metals of Group IA and the halogens of Group VIIA.
Solution 3
- Each of them can form a cation by loss of an electron.
H → H+ + e-
Li →Li+ + e-
- Both alkali metals and hydrogen act as reducing agents.
CuO + H2 → Cu + H2O
CuO + Na → Cu + Na2O
- Hydrogen burns in oxygen to form its oxide. It burns with a pop sound.
2H2 + O2 → 2H2O
Alkali metals also burn vigorously when heated in oxygen to form their respective oxides.
Lithium forms monoxide.
4Li + O2 → 2Li2O
- Hydrogen burns in oxygen to form its oxide. It burns with a pop sound.
2H2 + O2 → 2H2O
Alkali metals also burn vigorously when heated in oxygen to form their respective oxides.
Lithium forms monoxide.
4Li + O2 → 2Li2O
Solution 4
- Oxides of alkali metals are basic in nature, whereas the oxide of hydrogen H2O is a neutral oxide.
- Hydrogen atom has only one shell, but halogens have two or more shells.
Solution 5
- Hydrogen has one valence electron in its outermost orbit.
- Hydrogen burns in oxygen to form its oxide. It burns with a pop sound.
2H2 + O2 → 2H2O
- Hydrogen acts as a reducing agent.
CuO + H2 → Cu + H2O
Solution 6
Hydrogen was called inflammable air because of its combustible nature.
Solution 7
In the free state, hydrogen is found in traces in the earth's crust and atmosphere.
In the combined state, plant and animal tissues are made of compounds of hydrogen with carbon, oxygen and nitrogen.
Solution 8
- Like halogens (fluorine and chlorine), hydrogen too is a gas.
- Both show a tendency to form anions because they are one electron short of the nearest inert gas configuration.
H + e- → H-
Cl + e- →Cl-
- Both have valency 1.
- Hydrogen reacts with oxygen to form neutral oxide, H2O. Halogens react with oxygen to form acidic oxides like Cl2O and Cl2O7.
Solution 9
- Reactive metals such as potassium, sodium and calcium.
- Magnesium, aluminium, zinc and iron.
Solution 10
- 3Fe + 4H2O ⇋ Fe3O4 + 4H2
- The reaction is reversible because if hydrogen formed is not removed, then the iron oxide formed is reduced back to iron.
- Because the reaction is a reversible reaction, equilibrium is attained at 700°C. At this stage, the amount of reactants and products does not change.
Solution 11
They react with acids and can even react with hot concentrated alkalis to form hydrogen and a soluble salt.
Zn + 2NaOH → Na2ZnO2 + H2
2Al + 6NaOH→ 2Na2Al O3 + 3H2
Oxides and hydroxides of zinc and aluminium are amphoteric. They react with both bases and acids to give salt and water.
ZnO + 2HCl → ZnCl2 + H2O
ZnO + 2NaOH →Na2ZnO2 + H2O
Solution 12
- Fe +2 HC l → FeCl2 + H2
- Zn + 2NaOH → Na2ZnO2 + H2
- Pb + 2KOH → K2PbO2 + H2
- 2Al + 6NaOH→ 2Na2Al O3 + 3H2
Solution 13
- The reaction is highly exothermic and vigorous with the evolution of hydrogen.
2Na + 2H2O →2NaOH + H2
- Calcium sinks in water and the reaction is less vigorous.
Ca + 2H2O → Ca(OH)2 + H2
- Magnesium reacts slowly with boiling water and forms a base, magnesium hydroxide, liberating hydrogen gas.
Mg + 2H2O →Mg(OH)2 + H2O
- Magnesium burns in steam with an intense white light liberating hydrogen gas and white ash, i.e. magnesium oxide.
Mg + H2O → MgO + H2
Solution 14
- Iron is less reactive than zinc, but red hot iron reacts with steam, forming triferric tetra-oxide and hydrogen gas.
- 3Fe + 4H2O ⇋ Fe3O4 + 4H2
- If the product formed, i.e. hydrogen is not removed, then the iron oxide formed is reduced back to iron.
Solution 15
- Sodium
- Magnesium
- Zinc
- Calcium
Solution 16
- Sodium hydroxide + zinc → hydrogen + sodium zincate
- Calcium + water → calcium hydroxide + hydrogen
Study of the First Element - Hydrogen Exercise Ex. 6(B)
Solution 1
- Zn + HCl → ZnCl2 + H2
- Zn + 2NaOH → Na2ZnO2 + H2
- Zn + H2O → ZnO + H2
Solution 2
- Granulated zinc, dilute HCl or dil. H2SO4
- It is collected by the downward displacement of water.
- Zn + HCl → ZnCl2 + H2
Solution 3
- Hydrogen sulphide, sulphur dioxide, oxides of nitrogen, phosphine, arsine, carbon dioxide and water vapour are impurities present in the laboratory.
- The impurities can be removed from hydrogen by passing it through
1. Silver nitrate solution to remove arsine and phosphine.
AsH3 + 6AgNO3 → Ag3As + 3AgNO3 + 3HNO3
PH3 + 6AgNO3 → Ag3P + 3AgNO3 + 3HNO3
2. Lead nitrate solution to remove hydrogen sulphide.
Pb(NO3)2 + H2S → PbS + 2HNO3
3. Caustic potash solution to remove sulphur dioxide, carbon dioxide and oxides of nitrogen.
SO2 + 2KOH → K2SO3 + H2O
CO2 + 2KOH→ K2CO3+ H2O
2NO2 + 2KOH →KNO2 + KNO3 + H2O
4. A drying agent used to dry the gas. Common drying agents such as fused calcium chloride, caustic potash stick and phosphorus pentoxide remove water vapour.
So, the gas is purified and dried and then collected over mercury because mercury does not react with it.
Solution 4
Test: Collect some amount of gas in a test tube and take it to a flame.
If the gas burns quietly, then there is no more air in the flask.
Solution 5
Nitric acid is a powerful oxidising agent, and the oxygen formed due to its decomposition oxidises hydrogen to give water thus defeating the purpose of the reaction.
3Zn + 8HNO3 → 3Zn(NO3)2 + 4H2O + 2NO
Solution 6
Conc. sulphuric acid is not used in the preparation of hydrogen as it will produce sulphur dioxide.
Zn + 2H2SO4 →ZnSO4 + SO2 + 2H2O
Solution 7
- C + H2O
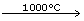 (CO + H2) - ∆
(CO + H2) - ∆
(CO + H2) + H2O ![]() CO2 + 2H2 + ∆
CO2 + 2H2 + ∆
- The mixture is passed through ammoniacal cuprous chloride solution in order to dissolve any uncombined carbon monoxide.
CuCl + CO + 2H2O →CuCl.CO.2H2O
Solution 8
- 3Fe + 4H2O⇋ Fe3O4 + 4H2
- Ca + 2H2O ⇋ Ca(OH)2 + H2
Solution 9
- Hydrogen gas. When red litmus is introduced in the solution, it turns blue.
- Ca + 2H2O → Ca(OH)2 + H2
- The solution turns blue.
- If dilute hydrochloric acid is added to the turbid solution, then they react and neutralise each other, forming the soluble salt calcium chloride (CaCl2) and water.
Ca(OH)2 + 2HCl → CaCl2 + 2H2O
Solution 10
- On heating thin strips of magnesium, copper and iron, they form oxides.
- Magnesium and iron react with HCl liberating hydrogen and forming their respective salts. Hydrogen cannot be prepared from metals which are below it in the activity series of metals (such as copper) because only metals which are more reactive than hydrogen can displace it from acids.
- Only magnesium will displace zinc from zinc sulphate solution because magnesium is more reactive than zinc in the activity series of metals. No reaction takes place in case of copper and iron because they are less reactive than zinc.
- Mg > Fe > Cu
Solution 11
- C. Mg
- C. Pd
- C. 1 n and 1 e-
- C. Al, Zn, Pb
Solution 12
- Zinc granules are preferred over pure zinc in the lab preparation of hydrogen because the impurity present in granulated zinc is copper, whose catalysing effect speeds up the rate of the reaction.
- Purified and dried hydrogen is collected over mercury because mercury has no reaction with it.
- The end of the thistle funnel should be dipped under acid so as to prevent the gas from escaping from the thistle funnel.
- Dilute sulphuric acid cannot be replaced by concentrated acid in the preparation of hydrogen because it is a strong oxidising agent and it will produce sulphur dioxide.
Study of the First Element - Hydrogen Exercise Ex. 6(C)
Solution 1
- In the free state, hydrogen is found in traces in the earth's crust and atmosphere. Volcanic gases contain 0.025%, the earth's crust 0.98%, the earth's atmosphere 0.01% and the atmosphere of the Sun and stars 1.1%.
- The name 'hydrogen' originated on account of its ability to form water.
Solution 2
- 2K + 2H2O →2KOH + H2
- Ca + 2H2O → Ca(OH)2 + H2
Solution 3
Metal preferred for collecting hydrogen from
- Cold water: Sodium
- Hot water: Magnesium
- Steam: Aluminium
Solution 4
- Zinc is the most preferred metal in the laboratory preparation of hydrogen.
- Dilute sulphuric acid.
Conc. nitric acid, even in its dilute form, is not used in the preparation of hydrogen from metals because it is a powerful oxidising agent and oxygen formed due to its decomposition oxidises hydrogen to give water, thus defeating the purpose of the reaction.
Conc. sulphuric acid is not used in the preparation of hydrogen as it will produce sulphur dioxide.
- The gas is collected by the downward displacement of water.
Common drying agents such as fused calcium chloride, caustic potash stick and phosphorus pentoxide remove water vapour.
Solution 5
- Calcium is expensive.
- Iron has to be heated, and hydrogen thus produced contains impurities such as hydrogen sulphide and sulphur dioxide.
- Aluminium forms a protective coating of Al2O3 due to its great affinity for oxygen. So, it does not give hydrogen with acid after the reaction has occurred for some time.
- Sodium reacts violently with acid.
Solution 6
Increasing order of reactivity of metals:
Iron < Zinc < Magnesium < Calcium < Sodium
Solution 7
Hydrogen is evolved when dilute HCl reacts with magnesium which is placed above hydrogen in the activity series. However, this does not occur for metals below hydrogen such as mercury and silver. This is because only metals which are more reactive than hydrogen can displace it from HCl.
Solution 8
- With metals:
3Fe + 4H2O ⇋ Fe3O4 + 4H2
- With non-metals:
Steam is passed over hot coke (1000°C) in furnaces of a special design called inverters giving water gas.
C + H2O ![]() (CO + H2) - ∆
(CO + H2) - ∆
Water is mixed with excess steam and passed over heated ferric oxide which acts as a catalyst and chromic oxide which acts as a promoter.
(CO + H2) + H2O ![]() CO2 + 2H2 + ∆
CO2 + 2H2 + ∆
The above mixture CO2 + H2 is formed through cold water under pressure (30 atm) or through caustic potash solution, which dissolves the more soluble carbon dioxide leaving hydrogen.
2KOH + CO2 →K2CO3 + H2O
The mixture is passed through ammoniacal cuprous chloride solution in order to dissolve any uncombined carbon monoxide.
CuCl + CO + 2H2O →CuCl.CO.2H2O
Solution 9
- Zn + H2SO4 → ZnSO4 + H2
- Fe + H2SO4 → FeSO4 + H2
Hydrogen cannot be prepared from metals which are below it in the activity series of metals such as copper because only metals which are more reactive than hydrogen can displace it from acids.
Solution 10
- It forms an insoluble coating of lead sulphate or lead chloride. So, further reaction is prevented.
- Potassium and sodium react violently with acid. Hence, potassium and sodium are not used for reaction with dilute hydrochloric acid or dilute sulphuric acid in the laboratory preparation of hydrogen.
Solution 11
NaOH and KOH
Zn + 2NaOH → Na2ZnO2 + H2
Zn + 2KOH → K2ZnO2 + H2
Metals such as zinc, lead and aluminium have a unique nature. They react with acids and can even react with hot alkalis to form hydrogen and a soluble salt.
Solution 12
- 2Na + 2H2O → 2NaOH + H2
- Ca + 2H2O → Ca(OH)2 + H2
- Mg + 2H2O → Mg(OH)2 + H2
- Zn + H2O → ZnO + H2
- 3Fe + 4H2O ⇋Fe3O4 + 4H2
- Zn + 2HCl → ZnCl2 + H2
- 2Al + 3H2SO4 →Al2(SO4)3 + 3H2
- Fe +2HCl →FeCl2 + H2
- Zn + 2NaOH → Na2ZnO2 + H2
- 2Al + 2KOH + 2H2O →2KAlO2 + 3H2
Solution 13
- Iron oxide is formed with the evolution of hydrogen gas.
- Hydrogen reduces heated magnetic oxide of iron.
Solution 14
- Magnesium
- Mg + 2H2O → Mg(OH)2 + H2
Mg + H2O →MgO + H2
Solution 15
On passing hydrogen gas through soap solution, soap bubbles filled with hydrogen fly high and burst. This behavior proves that hydrogen is lighter than air.
Solution 16
- Three volumes of hydrogen and one volume of nitrogen react at temperature 450-500°C and pressure 200-900 atm in the presence of finely divided iron catalyst with molybdenum as promoter to give ammonia.
N2 + 3H2⇋ 2NH3
- Equal volumes of hydrogen and chlorine react slowly in diffused sunlight to form hydrogen chloride.
H2 + Cl2 →2HCl
- Hydrogen gas on passing through molten sulphur reacts to give hydrogen sulphide.
H2 + S → H2S
- Hydrogen burns in the presence of electric spark with a 'pop' sound in oxygen and with a blue flame forming water.
2H2 + O2 →2H2O
Solution 17
- A = CuO, B = Cu
- Blue and red litmus paper when dipped in the colourless liquid do not change colour. This confirms the liquid formed is neutral and is water.
It changes white anhydrous copper sulphate to blue salt.
- Black copper oxide (A) on heating with hydrogen reduces copper oxide to reddish brown copper and itself gets oxidised to water.
Hydrogen is a strong reducing agent and removes oxygen from less active metals, i.e. it removes oxygen from heated metal oxides when passed over them and itself gets oxidised to water.
- CuO + H2
 Cu + H2O
Cu + H2O - Cu + HCl →No reaction
Copper is less reactive than hydrogen and hence cannot displace it from HCl.
Study of the First Element - Hydrogen Exercise Ex. 6(D)
Solution A
a. non-combustible
b. base
c. CuO
d. It is a strong oxidising agent.
e. (iii) PbO is oxidised to Pb.
f. (ii) Zn
g. (ii) Cu
Solution B. 1
- CuO, H2, H2O
- sparingly
- magnesium, iron and aluminium
- amalgam
- above, dilute hydrochloric, dilute sulphuric acid
Solution B. 2
- Chlorine
- MnO2
- H2S
- Hydrogen peroxide
- MnO2
Solution B. 3
- Hydrogen is separated from CO by passing the mixture through caustic potash solution.
- All metals above hydrogen in the activity series react with acids to give hydrogen.
- Hydrogen is dried by passing it through calcium chloride, caustic potash and phosphorous pentoxide.
- Very dilute nitric acid reacts with magnesium and manganese to produce hydrogen.
- Dil. H2SO4 reacts with zinc to liberate hydrogen.
Solution C. 1
- Zn + Pb2+ → Pb + Zn 2+
Zn → Zn 2+ + 2 e- ---- Oxidation
Pb2+ + 2 e → Pb ---- Reduction
- Zn + Cu2+ → Cu + Zn 2+
Zn → Zn 2+ + 2 e- ---- Oxidation
Cu2+ + 2 e → Cu ---- Reduction
- Cl2 + 2Br- → Br2 + 2Cl-
Cl2→ 2Cl- + 2 e- ---- Oxidation
2Br‒ + 2 e → Br2---- Reduction
Solution C. 2
- Equation in the ionic form:
Cu2+ SO42- + Fe → Fe2+ SO42- + Cu
- Fe → Fe2+ 2 e- ---- Oxidation
Cu2+ + 2 e → Cu ---- Reduction
Solution C. 3
- Na
The other metals react with dil. HCl liberating hydrogen gas, while sodium reacts violently with acid.
- NH3 is basic in nature.
- Cu
Metals more reactive than hydrogen can displace it from acids.
- Pb
Lead reacts with dilute sulphuric acid or HCl and forms an insoluble coating of lead sulphate or lead chloride.
The others react with dilute sulphuric acid or HCl to liberate hydrogen.
Solution C. 4
- Hydrogen is collected by the downward displacement of air because
i. It is insoluble in water.
ii. It forms an explosive mixture with air and therefore cannot be collected by the downward displacement of air even though it is lighter than it.
- Hydrogen is combustible, but it does not support combustion. So, the candle burns in air or oxygen when brought near the mouth of a jar containing hydrogen but is extinguished when pushed inside the jar as the supply of oxygen is cut off.
- Apparatus for laboratory preparation of hydrogen should be airtight and away from a naked flame because a mixture of hydrogen and air explodes violently when brought near a flame.
Solution C. 5
Half reaction:
A+ + e-→ A (Reduction)
B → B + e- (Oxidation)
a. A
b. B
c. B
Solution C. 6
- Oxidation
- Reduction
- Oxidation
- Oxidation
Solution C. 7
- PbO in the given reaction is reduced to Pb by losing oxygen.
- Magnesium undergoes oxidation by loss of electrons (Mg - 2e- → Mg2+).
- H2S undergoes oxidation by loss of hydrogen to give sulphur.
- Chlorine undergoes reduction by the addition of hydrogen to form HCl.
Solution D. 2
In the electronic concept, oxidation is a process in which an atom or ion loses electron(s).
Zn → Zn2+ + 2e-
Oxidation is also defined as a chemical process which involves
- Addition of oxygen
- Addition of electronegative ion
- Removal of hydrogen
- Removal of electropositive ion (element)
In the electronic concept, reduction is a process in which an atom or ion gains electrons.
Cu2+ + 2e-→ Cu
Reduction is also defined as a chemical process which involves
- Removal of oxygen
- Addition of electropositive ion
- Addition of hydrogen
- Removal of electronegative ion
Solution D. 1
In a chemical reaction, if one substance is oxidised, the other substance must necessarily be reduced. This is because the electrons lost during oxidation are simultaneously gained during reduction and vice versa.
For example: Zinc reacts with copper sulphate to form zinc sulphate and copper.
CuSO4 + Zn → ZnSO4 + Cu
Cu + 2SO42- + Zn →Zn + 2SO42- + Cu
Writing the half reaction,
Zn → Zn2+ + 2e- (Oxidation)
Cu2+ + 2e-→ Cu (Reduction)
They occur simultaneously as
Cu2+ + Zn→ Zn2++ Cu
Thus, oxidation and reduction always occur simultaneously.
Solution D. 3
Similarities between hydrogen and Alkali Metals
(1) Electronic configuration: both hydrogen and alkali metals have only one electron in their outermost orbits.
K L M N O P (shells)
H (1) : 1
Li (3) : 2, 1
Na (11) : 2, 8, 1
K (19) : 2, 8, 8, 1
Rb (37) : 2, 8, 18, 8, 1
Cs (55) : 2, 8, 18, 18, 8, 1
Fr : 2, 8, 18, 32, 18, 8, 1
(2) Valence electrons: All elements have one electron in their outermost orbit, i.e. their valency shell and so all of them have one valence electron.
(3) Valency: All alkali metals, including hydrogen, have valency 1.
(4) Ion formation: Each of them can form a cation i.e. positive ion, by loss of an electron.
H → H++ e-
Li → Li+ + e-
Na → Na+ + e-
As such all these elements have electropositive character.
(5) Reducing power: Both the alkali metals and hydrogen act as reducing agents.
CuO + H2 → Cu + H2O
CuO + 2Na → Cu + Na2O
(6) Burning: Hydrogen bums in oxygen to form its oxide (water).
2H2 + O2 → 2H2O
Hydrogen bunts with a pop sound.
Alkali metals also bum vigorously when heated in oxygen to form their respective oxides. Lithium form monoxide
4Li + O2 → 2Li2O
Sodium and hydrogen forms peroxide.
2Na + O2 → Na2O2
H2 + O2 → H2O2
While potassium, rubidium and caesium form superoxides having the general formula MO2 (where M stands for metal).
K + O2 → KO2
(7) Combination with nonmetals: Hydrogen as well as alkali metals react with non-metals like oxygen, sulphur and chlorine to form respective Compounds. Hydrogen - forms H2O; H2S; HCl
Sodium - forms Na2O; Na2S; NaCl
and so on.
Similarities between hydrogen and halogens
(1) Electronic configuration: Hydrogen and halogens have one electron less than the nearest inert gas.
H = 1 [He = 2],
F = 2, 7 [Ne = 2, 8]
Cl = 2. 8, 7 [Ar = 2, 8, 8]
(2) Valency : Both have valency 1.
Thus, they accept one electron to attain the electronic configuration of the nearest inert gas.
(3) Formation of ions: Both show a tendency to form anions since they are one electron shoe of the nearest inert gas configuration.
H + e- → H-
Cl + e- → Cl-
(4) Electronegative character: Both halogens and hydrogen are non-metals. They show electronegative character.
H + e- → H-
F + e- → F-
(5) Physical state: Like halogens (fluorine and chlorine), hydrogen too is a gas.
(6) Atomicity: Hydrogen as well as halogens exist in the form of diatomic molecules (H2, F2, Cl2, Br2, I2).
Solution D. 5
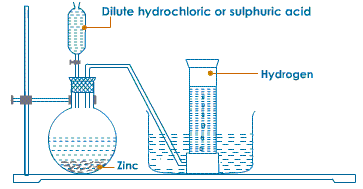
LABORATORY PREPARATION OF HYDROGEN
Reactants :
Granulated zinc; dilute hydrochloric acid or dilute sulphuric acid.
Procedure : Place some pieces of granulated zinc in a flat-bottom flask fitted with an air tight cork with two holes. Through one hole, pass a thistle funnel with a long stem provided with a stopper, and through the other, a long delivery tube (Fig. 6.6).
Pour dilute hydrochloric acid (or dilute sulphuric acid) through the funnel. Reaction :
Zn + 2HCl → ZnCl2 + H2↑
(dilute)
OR
Zn + H2SO4 → ZnSO4 + H2↑
(dilute)
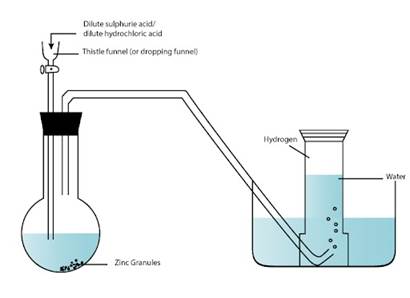
Observation :
Reaction will gradually start in the form of effervescence and evolution of gas. When all the air from the apparatus has been expelled, collect the gas over water.
Collection of hydrogen
Hydrogen is collected by the downward displacement of water because :
(i) it is virtually insoluble in water (20 mL of hydrogen dissolves in 1 litre of water under normal conditions).
(ii) it forms an explosive mixture with air and therefore cannot be collected by downward displacement of air even though it is lighter than air.
Impurities present with hydrogen :
(i) hydrogen sulphide (H2S)
(ii) sulphur dioxide (SO2)
(iii) oxides of nitrogen
(iv) phosphine (PH3)
(v) arsine (AsH3)
(vi) carbon dioxide
(vii) water vapour
Impurities can be removed from hydrogen by passing it through
1. Silver nitrate solution [to remove arsine and phosphine].
AsH3 + 6AgNO3 ⟶ Ag3As + 3AgNO3 + 3HNO3
PH3 + 6AgNO3 ⟶ Ag3P + 3AgNO3 + 3HNO3
2. Lead nitrate solution [to remove hydrogen sulphide].
Pb(NO3)2 + H2S ⟶ PbS + 2HNO3
3. Caustic potash solution [to remove sulphur dioxide, carbon dioxide and oxides of nitrogen].
SO2+ 2KOH ⟶ K2SO3 + H2O
CO2 + 2KOH ⟶ K2CO3 + H2O
2NO2 + 2KOH ⟶ KNO2 + KNO3 + H2O
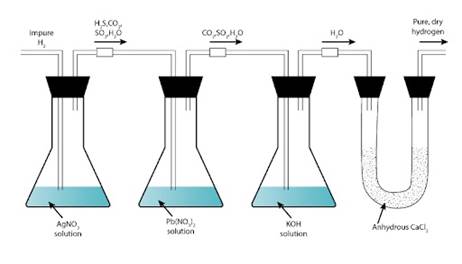
4. A drying agent is used to dry the gas. Common drying agents like fused calcium chloride, caustic potash stick and phosphorous pentoxide remove water vapour.
Thus the gas is purified and dried, and then collected over mercury. because mercury has no reaction with it.
Precautions :
The following precautions must be taken while preparing the gas because a mixture of hydrogen and air explodes violently when it is brought near a flame.
1. The apparatus should be airtight and there should be no leakage of gas.
2. There should be no flame burning near the apparatus.
3. The gas should be collected only after all the air in the apparatus has escaped. This can be ascertained by collecting some amount of gas in a test tube and taking it to a flame. If the gas bums quietly, then there is no more air in the flask.
4. The end of the thistle funnel should be dipped under acid so as to prevent gas from escaping from the thistle funnel.
Solution D. 4
Zinc grannules are preferred for this reaction over pure zinc because the impurity present in granulated zinc is copper, whose catalyzing effect speeds up the rate of reaction.
Solution D. 6
MANUFACTURE OF HYDROGEN
I. Bosch process :
Bosch process is used to prepare hydrogen on scale and it consists of the following steps :
(i) Steam is passed over hot coke (1000°C) in furnaces of a special design, called converters, giving water gas.
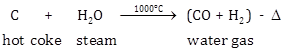
The reaction is endothermic.
(ii) Water gas is mixed with excess steam and passed over heated ferric oxide, which acts as a catalyst and chromic oxide Cr2O3, which acts as a promoter.
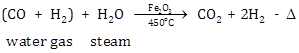
The reaction is exothermic.
(iii) Separation of carbon dioxide
Hydrogen can be separated from the mixture of CO2 and hydrogen by any one of the following methods.
(a) The above mixture, i.e. CO2 + H2, is forced through cold water under pressure (30 atm), which dissolves the more soluble carbon dioxide leaving behind hydrogen.
(b) Pass the mixture (CO2 + H2) through caustic potash solution, which removes carbon dioxide by reacting with it, leaving hydrogen.
2KOH + CO2 ⟶ KCO3 + H20
(iv) Separation of carbon monoxide
The mixture of water gas and steam (in step is passed through ammoniacal cuprous chloride solution in order to dissolve any uncombined carbon monoxide.
CuCl + CO + 2H2O ⟶ CuCl. CO. 2H2O
Thus, hydrogen gas is left behind.
II. By electrolysis of water*:
Commercially, hydrogen is also obtained by electrolysis of acidulated water.
Water is a poor conductor of electricity. In order to make it a conductor, a very small amount of less volatile acid, such as sulphuric acid (H2SO4), is added.
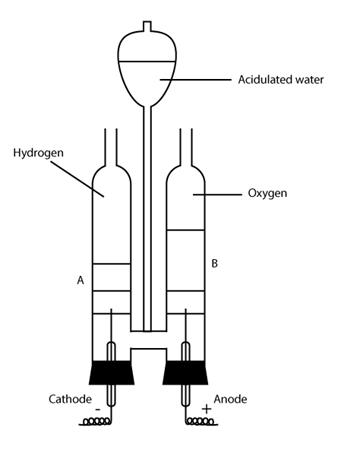
On passing electric current through acidulated water, water dissociates.
![]()
H+ being positively charged, moves towards the cathode (negatively-charged electrode).
At cathode H+ + e- ⟶ H
H + H ⟶ H2
Hydrogen gas is evolved at the cathode.
OH- being negatively charged, moves towards the anode (positively-charged electrode).
Anode OH- - e- ⟶ OH
OH + OH ⟶ H2O + 0
0 + O ⟶ O2
Oxygen is evolved at the anode.
Thus, water dissociates to give hydrogen and oxygen with the help of electric current.
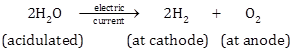
The main advantage of this process lies in the fact that oxygen is simultaneously produced at the anode and both the gases are pure.
This experiment proves that water is a compound made up of hydrogen and oxygen.
Solution E. 1
(a) The gas prepared by given method is Hydrogen gas, therefore 'A' is 'Hydrogen'.
(b) Hydrogen is collected by the downward displacement of water because:
(i) it is virtually insoluble in water (20 mL of hydrogen dissolves in 1 litre of water under normal conditions).
(ii) it forms an explosive mixture with air and therefore cannot be collected by downward displacement of air even though it is lighter than air.
(c) Nitric acid, even in its dilute form, is not used in the preparation of hydrogen from metals because:
Nitric acid is a powerful oxidizing agent, and the oxygen formed due to its decomposition oxidies the hydrogen to give water, thus defeating the purpose of the reaction.
3Zn + 8HNO3 → 3Zn(NO3)2 + 4H2O + 2NO↑
(d) Concentrated sulphuric acid is a good kdrying agent but it is not used to dry hydrogen as it reacts with hydrogen, thus defeating the purpose of the reaction.
H2SO4 + H2 → 2H2O + SO2

