Class 10 SELINA Solutions Chemistry Chapter 11 - Study of Compounds D. Sulphuric Acid
Study of Compounds D. Sulphuric Acid Exercise Intext 1
Solution 1
(a) Sulphuric acid is called King of Chemicals because there is no other manufactured compound which is used by such a large number of key industries.
(b) Sulphuric acid is referred to as Oil of vitriol as it was obtained as an oily viscous liquid by heating crystals of green vitriol.
Solution 2
(a) Two balanced equations to obtain SO2 is:
(i) 4FeS2 + 11O2 ![]() 2Fe2O3 +8SO2
2Fe2O3 +8SO2
(ii) S +O2 ![]() SO2
SO2
(b) The conditions for the oxidation of SO2 are:
(i) The temperature should be as low as possible. The yield has been found to be maximum at about 4100C-450oC
(ii) High pressure (2 atm) is favoured because the product formed has less volume than reactant.
(iii) Excess of oxygen increases the production of sulphur trioxide.
(iv) Vanadium pentoxide or platinised asbestos is used as catalyst.
(c) Vanadium pentoxide (V2O5)
(d) Sulphuric acid is not obtained directly by reacting SO3 with water because the reaction is highly exothermic which produce the fine misty droplets of sulphuric acid that is not directly absorbed by water.
(e)The chemical used to dissolve SO3 is concentrated sulphuric acid. The product formed is oleum.
Reaction involved in this process:
SO3 + H2SO4 → H2S2O7
Solution 3
Impurity of ARSENIC poisons the catalyst [i.e. deactivates the catalyst]. So, it must be removed before passing the mixture of SO2 air through the catalytic chamber.
Solution 4
(a) The catalyst which helps in the conversion of sulphur dioxide to sulphur trioxide in step C is Vanadium pentoxide.
(b) The two steps for the conversion of sulphur trioxide to sulphuric acid is:
(i) SO3 + H2SO4![]() H2S2O7
H2S2O7
(ii) H2S2O7 + H2O ![]() 2H2SO4
2H2SO4
(c)The substance that will liberate sulphur dioxide in step E is dilute H2SO4.
(d) The equation for the reaction by which sulphur dioxide is converted to sodium sulphite in step F is:
SO2+2NaOH![]() Na2SO3+H2O
Na2SO3+H2O
Or
Na2O+SO2![]() Na2SO3
Na2SO3
Study of Compounds D. Sulphuric Acid Exercise Ex. 11
Solution 1
Water is not added to concentrated acid since it is an exothermic reaction. If water is added to the acid, there is a sudden increase in temperature and the acid being in bulk tends to spurt out with serious consequences.
Solution 2
Balanced reactions are:
(a) Acidic nature:
(i) Dilute H2SO4 reacts with basic oxides to form sulphate and water.
2 NaOH+H2SO4 ![]() Na2SO4+2H2O
Na2SO4+2H2O
(ii) CuO+H2SO4 ![]() CuSO4+H2O
CuSO4+H2O
(iii) It reacts with carbonate to produce CO2.
Na2CO3+H2SO4![]() Na2SO4+H2O+CO2
Na2SO4+H2O+CO2 ![]()
(b) Oxidising agent:
H2SO4 ![]() H2O +SO2 +[O]
H2O +SO2 +[O]
Nascent oxygen oxidizes non-metals, metals and inorganic compounds.
For example,
Carbon to carbon dioxide
C+H2SO4 ![]() CO2 +H2O +2SO2
CO2 +H2O +2SO2
Sulphur to sulphur dioxide
S +H2SO4 ![]() 3SO2 +2H2O
3SO2 +2H2O
(d) Non-volatile nature:
It has a high boiling point (356oC) so it is considered to be non-volatile. Therefore, it is used for preparing volatile acids like hydrochloric acid, nitric acid from their salts by double decomposition reaction.
NaCl + H2SO4![]() NaHSO4 +HCl
NaHSO4 +HCl
KCl + H2SO4 ![]() KHSO4 +HCl
KHSO4 +HCl
Solution 3
(a) When dilute hydrochloric acid is treated with lead nitrate, lead chloride formed is soluble in hot water and when dilute sulphuric acid is treated with lead nitrate lead sulphate formed does not dissolve in hot water.
(b)
1. Dilute sulphuric acid treated with zinc gives Hydrogen gas which bums with pop sound.
Concentrated H2SO4 gives SO2 gas with zinc and the gas turns Acidified potassium dichromate paper green.
2.Barium chloride solution gives white ppt. with dilute H2SO4, This white ppt. is insoluble in all acids.
Concentrated H2SO4 and NaCl mixture when heated gives dense white fumes if glass rod dipped in Ammonia solution is brought near it.
Solution 4
(a) When sulphuric acid reacts with sulphur the product formed is Sulphur dioxide is formed.
S +2H2SO4 ![]() 3SO2 + 2H2O
3SO2 + 2H2O
(b) When sulphuric acid reacts with sodium hydroxide it neutralizes base to form sodium sulphate.
2NaOH + H2SO4 ![]() Na2SO4 + 2H2O
Na2SO4 + 2H2O
(c) When sulphuric acid reacts with sugar it forms carbon
C12 H22O11![]() 12C + 11H2O
12C + 11H2O
(d) When sulphuric acid reacts with carbon it forms carbon dioxide and sulphur dioxide gas.
C +2H2SO4 ![]() CO2 + 2H2O + 2SO2
CO2 + 2H2O + 2SO2 ![]()
(e) When sulphuric acid reacts with copper it forms copper sulphate and sulphur dioxide.
Cu + H2SO4 ![]() CuSO4 + 2H2O + SO2
CuSO4 + 2H2O + SO2 ![]()
Solution 5
(a) Concentrated sulphuric acid is hygroscopic substance that absorbs moisture when exposed to air. Hence, it is stored in air tight bottles.
(b) Sulphuric acid is not a drying agent for H2S because it reacts with H2S to form sulphur.
H2SO4+H2S ![]() 2H2O+SO2+S
2H2O+SO2+S ![]()
(c) Concentrated sulphuric acid has high boiling point (356oC). So, it is considered to be non-volatile. Hence, it is used for preparing volatile acids like Hydrochloric acid and Nitric acids from their salts by double decomposition.
NaCl+H2SO4![]() NaHSO4 +HCl
NaHSO4 +HCl
NaNO3 +H2SO4![]() NaHSO4 +HNO3
NaHSO4 +HNO3
Solution 6
(a) Due to its reducing property. i.e, it is a non-volatile acid.
NaCl+ H2SO4![]() NaHSO4 + HCl
NaHSO4 + HCl
(Conc.)
(b) It is a dehydrating agent.
HCOOH ![]() CO + H2O
CO + H2O
(c) Acidic property: Magnesium is present above hydrogen in the reactivity series so sulphuric acid is able to liberate hydrogen gas by reacting with magnesium strip.
Mg + H2SO4 ![]() MgSO4+H2
MgSO4+H2
(d) Due to its oxidizing character
Cu +H2SO4 ![]() CuSO4 +2H2O +SO2
CuSO4 +2H2O +SO2
(e) Due to its dehydrating property Hydrogen chloride gas is passed through concentrated sulphuric acid, it dries hydrogen chloride gas.
H2SO4 + H2O → H3O+ + HSO4−
(f)
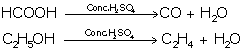
(i) Reaction with Ethanol - dehydrating agent
![]()
(ii) Reaction with Carbon - oxidising agent
![]()
Solution 7
The name of the salt of
(a) Hydrogen sulphites and Sulphites.
(b) Sulphate and bisulphate.
Solution 8
(a) Two types of salts are formed when sulphuric acid reacts with NaOH because sulphuric acid is dibasic.
NaOH + H2SO4 ![]() NaHSO4 + H2O
NaHSO4 + H2O
2NaOH + H2SO4![]() Na2SO4 + 2H2O
Na2SO4 + 2H2O
(b) A piece of wood becomes black when concentrated sulphuric acid is poured on it because it gives a mass of carbon.
(c) When sulphuric acid is added to sodium carbonate it liberates carbon dioxide which produces brisk effervescence.
Na2CO3+H2SO4![]()
![]() Na2SO4 +H2O+CO2
Na2SO4 +H2O+CO2 ![]()
Solution 9
|
Column 1 Substance reacted with acid |
Column 2 Dilute or concentrated acid |
Column 3 Gas |
| Zinc | Dilute sulphuric acid |
Hydrogen |
| Calcium carbonate | Concentrated sulphuric acid |
Carbon dioxide |
| Bleaching power CaOCl2 | Dilute sulphuric acid |
Only chlorine |
Solution 10
(i) Active metal + Acid ⟶ Metal sulphate + Hydrogen
(ii) Base + Acid ⟶ Salt + Water
(iii) Carbonate/hydrogen carbonate + Acid ⟶ Salt + Water + Carbon dioxide
(iv) Sulphide/hydrogen sulphite + Acid ⟶ Salt + Water + Sulphur dioxide
(v) Sulphide + Acid ⟶ Salt + Hydrogen sulphide
Solution 11(a)
Sulphuric acid is powerful dehydrating agent on account of its strong affinity towards water.
Solution 11(b)
Concentrated sulphuric acid as
i. Oxidising agent:
The oxidising property of conc. sulphuric acid its due to the fact that on thermal decomposition, it yeilds nacent oxygen [O].
H2SO4→ H2O + SO2 + [O]
ii. Non-volatile acid:
conc. sulphuric acid has high boiling point (338°C) that why it is said to be a non volitile compound, therefore it is used for preparing volatile acids like hydrochloric acids, nitric acids from there salts by double decomposition
H2SO4 + NaCl → NaHSO4 + HCl
Solution 12
(i) B
(ii) D
(iii) C
(iv) A
(v) A
Solution 13
(a) The acid formed when sulphur dioxide dissolves in water is sulphurous acid.
(b) Carbondioxide gas is released when sodium carbonate is added to solution of sulphur dioxide.
Solution 2008
a. (C) Lead nitrate
b. Liquid E is Ethanol.
c.
|
Name of process |
Inputs |
Catalyst |
Equation for catalyzed reaction output |
output |
|
Contact process |
Sulphur dioxide + oxygen |
Platinum or V2O5 |
2SO2 + O2 ⇄ 2SO3 |
Sulphuric acid |
d.
i. Zn + dil. H2SO4→ ZnSO4 + H2
ii. Na2CO3 + dil. H2SO4→ Na2SO4 + H2O + CO2
iii. Pb(NO3) + dil. H2SO4→ PbSO4 + 2HNO3
iv. Zn + dil. H2SO4→ ZnSO4 + H2
ZnSO4 + Na2CO3→ ZnCO3 + Na2SO4
e.
i. The property of concentrated sulphuric acid which allows it to be used in is used in the action when sugar turns black in its presence is its dehydrating property.
ii. The property of concentrated sulphuric acid which allows it to be used in the preparation of hydrogen chloride and nitric acid is its non-volatility.
H2SO4 + BaCl2 → BaSO4 + 2HCl
Solution 2009
Hydrogen Chloride
Solution 2010
a.
i. S + H2SO4 → 3SO2 +2H2O.
ii. C12H22O11 + Conc. H2SO4 → 6C + 6H2O
b. ZnO + H2SO4 → ZnSO4 + H2O.
c. C) Dilute sulphuric acid.
Solution 2011
a. Charring of sugar takes place. Sulphuric acid dehydrates sugar leaving behind carbon which is black in colour.
b. i. Hydrogen sulphide
c. i. ![]()
ii.
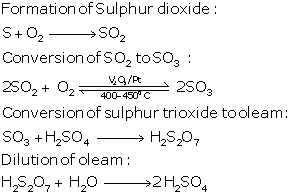
d.
i. Non-volatile nature
ii. as an oxidising agent
iii. dehydrating
Solution 2012
a. Hydrogen Sulphide (H2S).
b.
i. (B) Dehydrating agent
ii. (D) Oxidising agent
iii. (C) Non-volatile acid
iv. (A) Dilute acid
v. (D) Oxidising agent
c. ZnS + dil.H2SO4→ ZnSO4 + H2S
Solution 2013
a. when Conc. H2SO4 is added to a crystal of hydrated copper sulphate,it removes water of crystalisation from salt.
b. ii. Oxidising agent
c. C12H22O11 + Conc. H2SO4 → 6C + 6H2O
d. Sulphuric acid (H2SO4)
Solution 2014
a. C + H2SO4 → CO2 + 2H2O + 2SO2.
b. Sulphuric acid precipitates the insoluble sulphate of barium from the solution of barium chloride.
BaCl2 + H2SO4→ BaSO4 + 2HCl
Dilute HCl does not react with barium chloride solution, and thus, no precipitate is produced in the reaction.
c. Two conditions for the conversion of sulphur dioxide to sulphur trioxide is as follows:
Temperature: 450-500° C Catalyst: V2O5
![]()
d.
i. Dehydrating property of sulphuric acid:
H2SO4 has a great affinity for water, and therefore, it acts as a dehydrating agent.
ii. Acidic nature of sulphuric acid:
It acts as a strong dibasic acid.
H2SO4→ 2Hi+ + SO42-
It reacts with metals, metal oxides, metal hydroxides, carbonates etc. to form metallic sulphates and hydrogen at ordinary temperature.
Mg + H2SO4→ MgSO4 + H2↑
CuO + H2SO4→ CuSO4 + H2O
2NaOH + H2SO4→ Na2SO4 + 2H2O
ZnCO3 + H2SO4→ ZnSO4 + H2O + CO2↑
iii. As a non-volatile acid:
It has a high boiling point, so it is used to prepare volatile acids such as HCl, HNO3 and acetic acid from their salts.
NaCl + H2SO4→ NaHSO4 + HCl
NaNO3 + H2SO4→ NaHSO4 + HNO3
CH3COONa + H2SO4→ NaHSO4 + CH3COOH
Solution 2015
(a) In the contact process for the manufacture of sulphuric acid, the equations for the conversion of sulphur trioxide to sulphuric acid are
SO3 + H2SO4 → H2S2O7
(oleum or pyrosulphuric acid)
H2S2O7 + H2O → 2H2SO4
(b)
(i) Action of sulphuric acid on potassium hydrogen carbonate
2KHCO3+ H2SO4→ K2SO4+ 2H2O + 2CO2↑
(ii) Action of sulphuric acid on sulphur
S + 2H2SO4→ 3SO2 + 2H2O
(c)
(i) Concentrated sulphuric acid
(ii) Concentrated sulphuric acid

