Class 9 NCERT Solutions Chemistry Chapter 4 - Structure Of The Atom
Structure Of The Atom Exercise 39
Solution 1
Solution 2
Structure Of The Atom Exercise 41 - I
Solution 1
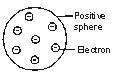
Thomson proposed a model of an atom. According to this model of an atom, an atom consists of a sphere of positive charge. The positive charge in the atom is spread all over like the red edible part of a watermelon, while the electrons are studded in the positively charged sphere, just like the seeds in the watermelon.
The negative and positive charges are equal in magnitude. These equal and opposite charges balance each other thus the atom becomes electrically neutral as a whole.
Concept insight: Recall the charges on subatomic particles.
Solution 2
Concept insight: Recall the Rutherfors's model of an atom.
Solution 3
Solution 4
If a foil of a heavy metal like platinum is used, then the observations in the alpha-particle scattering experiment would be the same as that in the gold foil experiment.
If a foil of a light metal like lithium is used, then the observations in the alpha-particle scattering experiment would not be the same as that in the gold foil experiment.
Structure Of The Atom Exercise 41 - II
Solution 1
(i) Electrons
(ii) Protons and
(iii) Neutrons
Solution 2
No. of protons = 2
As atomic mass = no. of protons + no. of neutrons
No. of neutrons = At. mass - no. of protons
= 4 - 2 = 2
Structure Of The Atom Exercise 42
Solution 1
|
Element |
At no |
Electronic configuration |
||
|
K shell |
M shell |
L shell |
||
|
Carbon Sodium |
6 11 |
2 2 |
4 8 |
1 |
Concept insight: Recall that the atomic number of sodium is 11 and that of carbon is 6.
Solution 2
Structure Of The Atom Exercise 44 - I
Solution 1
Chlorine: At. No. of Cl = 17
Its electronic configuration = 2, 8, 7
Valency of Cl = 8 7 = 1
Sulphur: At. no. of S = 16
Its electronic configuration = 2, 8, 6
Valency of S = 8 6 = 2
Magnesium:
At. no. of Mg = 12
Its electronic configuration = 2, 8, 2
Valency of Mg = 2
Concept insight: As we know, when the outermost shell of an atom contains 4 or less than 4 electrons, its valency is equal to the number of valence electrons in the outermost shell and when the outermost shell contains more than 4 electrons, valency of the atom is equal to 8 - no. of valence electrons in the atom.
Structure Of The Atom Exercise 44 - II
Solution 1
(i) Atomic number = Number of Protons = 8
(ii) In the given atom, total number of positive charge is equal to the total number of negative charge.
Number of Protons (8) = Number of electrons (8)
So, the charge on the atom will be zero.
Solution 2
Mass number = Number of Protons + Number of neutrons
So, Mass number of oxygen = 8 + 8 = 16
Mass number of sulphur = 16 + 16 = 32
Structure Of The Atom Exercise 45
Solution 1
H, D and T are the three isotopes of hydrogen with same atomic number and different mass numbers of 1, 2 and 3 respectively.
|
Element |
Symbols |
Electrons |
Protons |
Neutrons |
|
Hydrogen Deuterium Tritium |
H D T |
1 1 1 |
1 1 1 |
0 1 2 |
Concept insight: Recall the mass numbers of each of the isotopes.
Solution 2
|
Isotopes |
Protons |
Electrons |
Neutrons |
ElectronicsConfiguration |
|
Shell |
||||
|
K L M N |
||||
|
3517Cl 3717Cl |
17 17 |
17 17 |
18 20 |
2 8 7 - 2 8 7 - |
|
Isobars |
Protons |
Electrons |
Neutrons |
ElectronicsConfiguration |
|
Shell |
||||
|
K L M N |
||||
|
4020Ca 4018Ar |
20 18 |
20 28 |
20 22 |
2 8 8 2 2 8 8 - |
Concept insight: Recall the definitions of isotopes and isobars.
Structure Of The Atom Exercise 46
Solution 1
|
Particle |
Nature of charge |
Mass |
Location |
|
Electron Proton Neutron |
negative |
9.0 1.672 1.672 |
Extra nuclear part Nucleus Nucleus |
Concept insight: This question is very important from exam point of view.
Solution 2
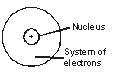
J.J. Thomson attributed the mass of an atom due to electrons and protons which are evenly spread throughout the atom. But this did not agree with observations of Rutherford according to whom the mass is concentrated in a very small space later called nucleus.
Thomson's model of the atom could not explain the results of alpha particle scattering experiment carried out by Rutherford.
Concept insight: Recall the features of J. J. Thomson's model of the atom.
Solution 3
The major limitation of Rutherford's model of the atom is that it does not explain the stability of the atom. As we know now, when charged bodies move in circular motion, they emit radiations. This means that the electrons revolving round the nucleus (as suggested by Rutherford) would lose energy and come closer and closer to nucleus, and a stage will come when they would finally merge into the nucleus. This makes the atom unstable, which is clearly not the case. The electrons do not fall into the nucleus, atoms are very stable and do not collapse on their own.
Concept insight: Recall the features of Rutherford's model of the atom.
Solution 4
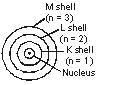
1. An atom consists of a small positively charged nucleus at its centre.
2. The whole mass of the atom is concentrated at the nucleus.
3. The volume of nucleus is smaller than the volume of the atom (by a ratio of about 1 : 105).
4. The protons and neutrons of the atom are present in the nucleus.
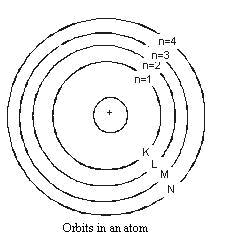
5. The electrons of the atom, which are negatively charged, revolve around the nucleus in definite circular paths known as orbits or which are designated as K, L, M, N etc. or numbered as (n) = 1, 2, 3, 4 etc. (outward from the nucleus).
6. As each orbit is associated with a fixed amount of energy, these orbits are also known as energy levels.
7. While revolving in diserete orbits, the electrons do not radiate energy. But when an electron jumps from one energy level to another, the energy of the atom changes.
Solution 5
|
Feature |
Thomson's model of an atom |
Rutherford's model of an atom |
Bohr's model of an atom |
|
1. Positive Charge (Protons)
2. Negative charge
3. Diagrammatic representation
4. Limitation: |
As per Thomson's model of an atom, an atom consists of a positively charged sphere.
The electrons are embedded in the positively charged sphere of an atom, like the seeds in a watermelon.
This model could not explain the results of alpha particle scattering experiment carried out by rutherford. |
The positive charge is concentrated at the core of the atom, which is called nucleus.
This model could not explain the stability of the atom. |
The positive charge is present in the core of the atom, called nucleus.
Advantage: This model explains the stability of atoms. |
Solution 6
1. The maximum number of electrons which a shell can have is represented by 2n2, where n is the quantum number of that particular energy shell. Thus, the maximum number of electrons in the first four shells are :
1st (K) shell 2
2nd (L) shell 2
3rd (M) shell 2
4th (N) shell 2
4. Electrons are not accommodated in a given shell unless the inner shells are filled, i.e., the shells are filled in a step-wise manner.
Solution 7
Valency is defined as the combining capacity of an atom of an element. If an atom has 4 or less than 4 electrons in its valence shell, then valency is equal to the no. of valence electrons. But if it has more than 4 valence electrons, then valency is equal to 8 - no. of valence electrons.
Silicon has atomic number 14 and its electronic configuration is:
|
K |
L |
M |
|
2 |
8 |
4 |
So, valency of silicon = 8 - 4 = 4
Oxygen has atomic number 8 and its electronic configuration is:
|
K |
L |
|
2 |
6 |
So, valency of oxygen = 8 - 6 = 2
Concept insight: Remember that the atomic number of oxygen is 8 and Si is 14.
Structure Of The Atom Exercise 47
Solution 8
(i) Atomic number: Atomic number of an atom is the total number of protons present within the nucleus of an atom is known as atomic number.
Example: As sodium atom has 11 protons in its nucleus, its atomic number is 11.
(ii) Mass number: Mass number of an atom is the sum total of the masses of all the nucleons present in the nucleus of an atom, i.e.,
Mass Number = No. of Protons + No. of Neutrons
Example: As a sodium atom has 11 protons and 12 neutrons in its nucleus, its mass number = 11 + 12 = 23.
(iii) Isotopes: Isotopes are the atoms of the same element having same atomic number but different mass number.
Example: Hydrogen has three isotopes 11H, 12H, 13H. The atomic number of all the three is 1, but their mass numbers are 1, 2 and 3 respectively.
(iv) Isobars: Isobars are the atoms of different elements having the same mass number but different atomic numbers.
Example: Mass numbers of calcium and argon atoms are 40, but different atomic numbers 20 and 18 respectively.
Two uses of isotopes are:
(i) An isotope of uranium is used as a fuel in nuclear reactors.
(ii) An isotope of cobalt is used in the treatment of cancer.
Concept insight: Definitions and examples are important from exam point of view.
Solution 9
Atomic number of Na = 11
No. of electrons in Na atom = 11
So, No. of electrons in Na+ ion = 11 1 = 10
Hence, electronic configuration of Na+ = 2, 8
In Na+, K and L shells are completely filled since K shell can have a maximum of 2 electrons and L shell can have a maximum of 8 electrons.
Concept insight: Remember that when a cation is formed, an electron is removed from the outermost shell of the atom
Solution 10
Solution 11
Solution 12
Concept insight: This numerical is important from exam point of view.
Solution 13
As we know, mass number of an atom = No. of protons + No. of Neutrons
So, Mass number of X = 6 + 6 = 12
Mass number of Y = 6 + 8 = 14
As both X and Y have the same atomic number (6) but different numbers (i.e., 12 and 14 repectively), so they are isotopes.
Concept insight: This numerical is important from exam point of view.
Solution 14
(a) F
(b) F
(c) T
(d) T
Solution 15
Solution 16
(b)
(c)
(d)
Solution 17
Structure Of The Atom Exercise 48
Solution 18
Solution 19
First row:
Since atomic no. is 9 so, the element is Fluorine.
Atomic no. = No. of protons = no. of electrons = 9
Mass number = no. of protons + no. of neutrons = 9 + 10 = 19
Second row:
Since atomic no. is 16 so, no. of protons = no. of electrons = 16
No. of neutrons = Mass no. - no. of protons = 32 - 16 = 16
Third row:
No. of protons = Atomic no. = 12
So, the element is Magnesium.
No. of electrons = no. of protons = 12
No. of neutrons = Mass no. - no. of protons = 24 - 12 = 12
Fourth row:
No. of protons = Atomic no. = 1
So, the element is Deuterium.
No. of electrons = no. of protons = 1
No. of neutrons = Mass no. - no. of protons = 2 - 1 = 1
Fifth row:
No. of protons = Atomic no. = 1
The element is Protium since the mass number is 1.
|
Atomic number |
Mass number |
Number of neutrons |
Number of protons |
Number of electrons |
Name of the atomic species |
|
9 16 12 1 1 |
19 32 24 2 1 |
10 16 12 1 0 |
9 16 12 1 1 |
9 16 12 1 0 |
Fluorine Sulphur Magnesium Deuterium Protium |
Concept insight: For asnwering this question, recall the definitions of atomic number and mass number.

 10-19 C
10-19 C