Class 10 SELINA Solutions Chemistry Chapter 13 - Practical Work
Practical Work Exercise Intext 1
Solution 1
(a)(i) Chemical test for ammonia:
If a rod dipped in concentrated hydrochloric acid is brought near ammonia gas, dense white fumes of ammonium chloride (NH4Cl) are formed.
(ii) Chemical test for Sulphur dioxide:
It decolorizes pink coloured potassium permanganate solution.
![]()
(iii) Chemical test for HCl:
When HCl gas is passed through AgNO3 solution, white precipitates of AgCl are formed which gets dissolved in excess of NH4OH.
![]()
(iv) Chemical test for Chlorine:
It turns moist starch iodide paper (KI + starch solution) blue black.
(v) Chemical test for Carbon dioxide:
When this gas is passed through lime water, it turns milky due to the formation of white precipitates of CaCO3 and on passing excess of carbon dioxide gas, this milkiness disappears.
(vi) Chemical test for oxygen:
This gas is absorbed in colourless alkaline solution of pyrogallol and turns it dark brown.
(vii)Chemical test for hydrogen:
It burns with a pop sound when a burning taper is brought near it.
(b)Ammonia is a basic gas and its basic nature is suspected through litmus paper test because it changes the colour of red litmus paper to blue.
(c)Chlorine, carbon dioxide, hydrogen chloride, hydrogen sulphide and sulphur dioxide are acidic gases since they convert blue litmus to red.
(d)A is chlorine and B is Sulphur dioxide.
(e)Water vapour.
Solution 2
(a)O2
(b)NH3, HCl, SO2, H2S, CO2, NO2, Cl2
(c)Water vapour
(d)SO2
Solution 3
(a)Na2CO3 and K2CO3
(b)SO2
(c)CO2
(d)Cl2
(e)H2S
Practical Work Exercise Ex. 13
Solution 1
Silver nitrate and ammonium nitrate.
Solution 2
(a)Cl-
(b)SO42-
(c)CO32-
(d)SO32-
Solution 3
(a)Since the salt solution turned blue litmus red hence the salt may be an acid.
(b)Since addition of barium chloride into the solution of salt gave white precipitate so the salt may contain SO42-, SO32-, CO32- anion.
(c)The flame test of the salt gives persistent golden yellow colourisation which suggests presence of Na+ ion.
Solution 4
(a) Ca2+
(b)Cu+
(c)The three ways are:
i.Ammonia gas turns moist red litmus blue.
ii.If a rod dipped in concentrated HCl is brought near the gas, dense white fumes of NH4Cl are formed.
iii.The gas turns colourless Nessler's reagent i.e. K2HgI4 brown.
Solution 5
|
Hydrogen sulphide |
Ammonia |
Sulphur dioxide |
Hydrogen chloride |
|
|
Shake the gas with red litmus solution |
No change in the colour of litmus solution |
Red litmus solution becomes blue in colour. |
No change in the colour of litmus solution |
No change in the colour of litmus solution |
|
Shake the gas with blue litmus solution |
Blue litmus solution becomes red in colour. |
No change in the colour of blue litmus solution. |
Blue litmus solution becomes red in colour. |
Blue litmus solution becomes red in colour |
|
Apply a burning splint to a gas |
No reaction. |
No reaction. |
No reaction. |
No reaction. |
Solution 6
(P)Ammonium chloride
(Q)Calcium
(R)Calcium hydroxide
(S)Lead (II) Nitrate
(T)Calcium Oxide
(U)Lead (II) Oxide
(V)Chlorine
(W)Hydrogen chloride
Solution 7
|
Carbonate |
Colour of residue on cooling |
|
Zinc Carbonate |
white |
|
Lead Carbonate |
yellow |
|
Copper Carbonate |
black |
Solution 8
(a)Sodium carbonate and sodium sulphite can be distinguished by using acidified K2Cr2O7:
Take a small quantity of salt in a test tube; add dil. H2SO4.and warm if necessary. Now if on bringing a filter paper moistened with acidified K2Cr2O7 near the gas evolved, the orange colour of the paper turns green then it is sodium sulphite.
(b)Sodium chloride and sodium sulphide:
The salts can be distinguished by using silver nitrate. Curdy white precipitate is insoluble in dil. HNO3 but dissolves in NH4OH then chloride is present.
If sulfide ions are present in the solution, as they would be in a solution of sodium sulfide, they will react with the silver ions to produce a black precipitate. This black precipitate is silver sulfide.
(c)Sodium hydroxide solution and ammonium hydroxide solution:
These salts can be distinguished by using a metal cation like calcium. When we add calcium salt to sodium hydroxide and ammonium hydroxide, then a white curdy ppt. is formed only in case of sodium hydroxide.
(d)Ammonium sulphate and sodium sulphate:
These salts can be distinguished by using KOH. When KOH is added to ammonium sulphate, ammonia gas is evolved. Whereas there is no evolution of ammonia gas in case of sodium sulphate.
(e)Add barium chloride solution to sulphuric acid, nitric acid and hydrochloric acid. A white precipitate is formed in dilute sulphuric acid, and no such precipitate is formed in nitric acid and hydrochloric acid.
BaCl2(aq) + H2SO4(aq) → BaSO4(s) + 2HCl(aq)
(f) Magnesium chloride and magnesium nitrate solutions:
Chloride and nitrate anion can be distinguished by addition of conc. H2SO4 solution to the salt solutions. Evolution of colourless gas with pungent smell indicates the presence of chloride ions while the evolution of reddish brown fumes which become thick on adding copper turnings indicates the presence of nitrate ions.
Solution 9
|
Aqueous salt solution |
Colour of the precipitate when NaOH is added in small quantity |
Nature of the(soluble or insoluble) when NaOH is added in excess |
|
copper (II) sulphate zinc nitrate
lead nitrate calcium chloride iron (III) sulphate |
(i)Pale blue
(ii) White gelatinous (iii)White chalky (iv)White curdy (v) Reddish brown
|
(vi)Insoluble
(viii)Soluble
(viii)Soluble (ix)Insoluble (x)Insoluble |
Solution 10
(a)Lead chloride as precipitate and sodium nitrite are formed.
(b)
|
Zinc chloride |
Zinc nitrate |
Zinc sulphate |
|
|
Barium chloride |
No reaction |
No reaction |
White ppt. is obtained |
|
Lead nitrate |
No reaction |
No reaction |
No reaction |
(c)Dilute sulphuric acid liberates carbon dioxide from metallic carbonates and bicarbonates. Carbon dioxide when bubbled into a test tube containing calcium hydroxide solution turns it milky.
(d) On strong heating, the light amorphous white solid of zinc carbonate, changes to pale yellow.
Gives off a colourless and odourless gas that turns lime water milky. The milkiness disappears on passing excess of gas.
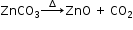
The gas has no effect on acidified K2Cr2O7 or acidified KMnO4.
The residue, on cooling, changes to a white colour.
i.e. residue is yellow when hot and white when cold.
Residue is zinc oxide.
Gas evolved is carbon dioxide.
Solution 11
(I)Iron (II) Sulphate and Magnesium sulphate
(II)Iron (III) chloride and Zinc Chloride
(III)Lead nitrate
(IV)Copper nitrate.
(V)Lead nitrate.
Solution 12
|
Solution |
Acids |
Alkalies |
|
(a) Alkaline phenolphthalein solution (b) Methyl orange solution (c) Neutral litmus solution |
Colourless Pink Red |
Pink Yellow Blue |
Solution 13
|
Salt |
Anion |
|
A |
Cl- |
|
B |
S2- |
|
C |
NO3- |
|
D |
SO32- |
|
E |
CO32- |
Solution 14
(a) (ii) Iron[II] sulphate
(b) (i) Gas A is ammonia gas.
(ii) Gas C is hydrogen sulphide.
(iii) Gas D is sulphur dioxide.
Solution 15
|
|
Column I |
|
Column II |
|
(a) |
Hydrogen sulphide |
D. |
Turns moist lead acetate paper silvery black |
|
(b) |
Nitric oxide |
C. |
Turns reddish brown when it reacts with oxygen |
|
(c) |
Carbon dioxide |
B. |
Turns lime water milky |
|
(d) |
Sulphur dioxide |
A. |
Turns acidified potassium dichromate solution green |
Solution 2009
(a) (iii) Acidified potassium dichromate paper
(b) R is ferrous sulphate.
(c)
i. When BaCl2 solution is added to the given solution, ZnSO4 gives a white ppt. of barium sulphate, while no ppt. is obtained with ZnCl2 solution.
ii. When NaOH solution is added to the given solution, iron (II) chloride gives a dirty green ppt. of ferrous hydroxide, while reddish brown ppt. of iron(III) hydroxide is obtained with iron (III) chloride.
iii. When AgNO3 solution is added to the given solution, CaCl2 solution gives a white ppt., while no change is observed with calcium nitrate solution.
Solution 2010
(a)
(i) C. Chromium sulphate
(ii) A. Nitroso iron (II) sulphate
(b) (i) Litmus turns blue to red, and then gets bleached.
(ii) Paper turns from pink to white.
(c) MgSO4 + BaCl2→ MgCl2 + BaSO4
Solution 2011
(a) Barium chloride
Solution 2012
(a) Sulphur dioxide
(b) (i) Nitrate ion, NO3-
(ii) Chloride ion, Cl-
(iii) Carbonate ion, CO32-
(iv) Sulphate ion, SO42-
(c)
i. On adding dil. sulphuric acid to sodium carbonate, a colourless and odourless gas is evolved which has no action on acidified potassium dichromate paper.
Na2CO3+ H2SO4→ Na2SO4+ H2O + CO2
Sodium sulphite on adding dil. sulphuric acid liberates a colourless gas having burning sulphur smell and turns acidified potassium dichromate paper from orange to green.
Na2SO3 + H2SO4→ Na2SO4 + H2O + SO2
K2Cr2O7 + 3SO2 + H2SO4 → K2SO4 + Cr2(SO4)3 + H2O
(Orange) (Green)
ii. Ferrous nitrate on reaction with little NaOH produces a dirty green precipitate which is insoluble in excess. Lead nitrate on reaction with little NaOH produces white precipitate which is soluble in excess.
iii. Add a little dil. sulphuric acid to copper oxide and manganese dioxide and heat gently. The copper oxide reacts to produce a blue solution of copper sulphate. The manganese dioxide gives a colourless solution.
CuO + H2SO4 → CuSO4 + H2O
(Blue)
MnO2 + 2H2SO4 → MnSO4 + 2H2O
(Colourless)
Solution 2013
(a)
i. Add silver nitrate solution to both solutions. Sodium chloride will form a curdy white ppt., whereas sodium nitrate will not undergo any reaction.
ii. Hydrogen chloride gas gives thick white fumes of ammonium chloride when a glass rod dipped in ammonia solution is held near the vapour of the acid, whereas no white fumes are observed in case of hydrogen sulphide gas.
iii. Calcium nitrate forms no ppt. even with addition of excess of NH4OH, whereas zinc nitrate forms a white gelatinous ppt. which dissolves in excess of NH4OH.
iv. Carbon dioxide gas has no effect on acidified KMnO4 or K2Cr2O7, but sulphur dioxide turns potassium permanganate from pink to colourless.
Solution 2014
(a) Sodium nitrate on treatment with dilute sulphuric acid gives sodium bisulphate and nitric acid.
NaNO3 + H2SO4 →NaHSO4 + HNO3
Sodium sulphite on treatment with dilute sulphuric acid gives sodium sulphate and sulphur dioxide.
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2
(b) When moist starch iodide paper is introduced into chlorine gas, chlorine oxidises iodide to iodine, which shows up as blue when complexed with starch.
(c) On carrying out the flame test with a salt P, a brick red flame is obtained. Hence, the cation P is Ca2+.
(d) Gas Q turns moist lead acetate paper silvery black. Hence, the gas is H2S.
(e) pH of liquid R is 10. Hence, substance R is a base.

