Class 10 SELINA Solutions Chemistry Chapter 12 - Organic Chemistry
Organic Chemistry Exercise Intext 1
Solution 1
(a) Organic chemistry may be defined as the chemistry of hydrocarbons and its derivatives.
(b) Vital Force Theory is a theory made by the Scientist Berzelius in 1809 which assumed that organic compounds are only formed in living cells and it is impossible to prepare them in laboratories.
It was discarded because Friedrich Wohler showed that it was possible to obtain an organic compound(urea) in the laboratory.
Solution 2
(a) Few sources of organic compounds are:
Plants
Animals
Coal
Petroleum
Wood
(b) The various applications of organic chemistry is:
It is used in the production of soaps, shampoos, powders and perfumes.
Various fuels like natural gas, petroleum are also organic compounds.
The fabrics that we use to make various dresses are also made from organic compounds.
Solution 3
Organic compounds are present everywhere. They are present in:
It is present in the production of soaps, shampoos, powders and perfumes.
It is present in the food we eat like carbohydrates, proteins, fats, vitamins etc.
Fuel like natural gas, petroleum are also organic compounds.
Medicines, explosives, dyes, insecticides are all organic compounds.
Thus we can say that organic compounds play a key role in all walks of life.
Solution 4
The unique properties shown by carbon are:
Tetravalency of carbon
Catenation
Isomerism
Solution 5
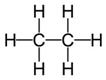
(a) Tetravalency: Carbon can neither lose nor gain electrons to attain octet. Thus it shares four electrons with other atoms. This characteristics of carbon by virtue of which it forms four covalent bonds, is called Tetravalency of carbon.
In structural form :
![]()
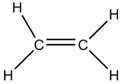
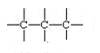
(b) Catenation: The property of self -linking of atoms of an element through covalent bonds in order to form straight chains, branched chains and cyclic chains of different sizes is known as catenation.
Carbon- carbon bond is strong so carbon can combine with other carbon atoms to form chains or rings and can involve single, double and triple bonds.
Solution 6
Four properties of organic compound that distinguish them from inorganic compounds are:
(i) Presence of carbon.
(ii) Solubility in the organic solvents.
(iii) Forming of covalent bonds.
(iv) Having low melting and boiling points.
Solution 7
Due to the unique nature of carbon atom, it gives rise to formation of large number of compounds. Thus this demands a separate branch of chemistry.
Solution 8
Hydrocarbons are compounds that are made up of only carbon and hydrogen.
Comparison of saturated and Unsaturated hydrocarbons:
|
Saturated Hydrocarbon |
Unsaturated Hydrocarbon |
|
1. Carbon atoms are joined only by single bonds. |
Carbon atoms are joined by double or by triple bonds. |
|
2. They are less reactive due to the non-availability of electrons in the single covalent bond. |
They are more reactive due to presence of electrons in the double or the triple bond. |
|
3. They undergo substitution reaction. |
They undergo addition reaction. |
Solution 9
Due to presence of unique properties of carbon like Tetravalency, catenation and Isomerism large number of organic compounds are formed.
Solution 10
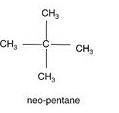
(a) Single Bond compound: For example: In pentane
![]()
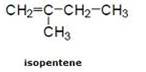
(b) Double bond compound: For example:- In pentene
![]()
![]()
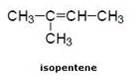
(c) Triple bond compound: In case of Hexyne:
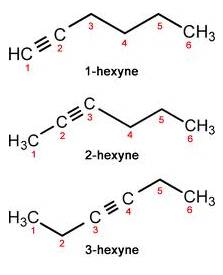
Solution 11
(a) Cyclic compound with single bond: cyclopentane
Structure:
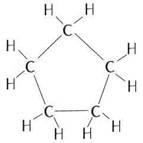
(b) Cyclic compound with triple bond: cyclopentyne
Structure:
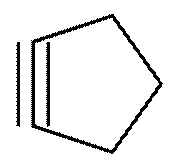
Solution 12
The member of each of the following is:
(a) Saturated Hydrocarbon: Hexane (C6H14)
(b) Unsaturated Hydrocarbon: Hexene (C6H12)
Solution 13
Substitution reaction: A reaction in which one atom of a molecule is replaced by another atom (or group of atoms) is called a substitution reaction.
Addition reaction: A reaction involving addition of atom(s) or molecules(s) to the double or the triple bond of an unsaturated compound so as to yield a saturated product is known as addition reaction.
Organic Chemistry Exercise Intext 2
Solution 1
A functional group is an atom or a group of atoms that defines the structure (or the properties of a particular family) of organic compounds.
The structural formula of
(a) Halides :-R-X
Example:
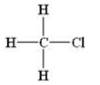
(b)Alcohols:- R-OH
Example:
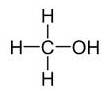
(c) Aldehydes:-R-CH=O
Example:
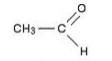
Solution 2
(a) A homologous series is a group of organic compounds having a similar structure and similar chemical properties in which the successive compounds differ by a CH2 group.
(b) The difference in molecular formula of any two adjacent homologues is
(i) It differs by 14 a.m.u in terms of molecular mass.
(ii) It differs by three atoms. The kind of atoms it differs is one carbon and two hydrogen.
Solution 3
a. Butyne; its formula is C4H6.
b. Butanol; its formula is C4H9OH.
Solution 4
(i) Physical properties: The alkyl group determines the physical properties.
(ii) Chemical properties: The functional group is responsible for the chemical properties.
Solution 5
The alkyl radical and the functional group are:
|
Sr.No |
Formula |
Name of alkyl radical |
Name of Functional group |
|
a |
CH3OH |
Methyl |
Alcohol |
|
b |
C2H5OH |
Ethyl |
Alcohol |
|
c |
C3H7CHO |
Propyl |
Aldehyde |
|
d |
C4H9COOH |
Butyl |
Carboxyl |
|
e |
CH3COOH |
CH3 |
COOH |
|
f |
HCHO |
H |
CHO |
Solution 6
(a) An alkyl group is obtained by removing one atom of hydrogen from an alkane molecule. Alkyl group is named by replacing the suffix 'ane' of the alkane with the suffix -yl.
(b) The name of three alkyl radicals are:
Methyl
Ethyl
Propyl
They are formed by removing 1 hydrogen from an alkane.
CH4![]() -CH3+H+
-CH3+H+
Methyl
CH3-CH3 ![]() CH3-CH2 -+ H+
CH3-CH2 -+ H+
Ethyl
CH3-CH2-CH3 ![]() CH3-CH2-CH2 -+ H+
CH3-CH2-CH2 -+ H+
Propyl
Solution 7
The names and the structural formula of first three members of the homologous series of alkane are:
(i)
CH4Methane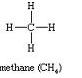
(ii)
C2H6Ethane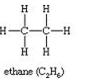
(iii)
C3H8Propane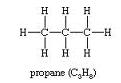
Organic Chemistry Exercise Ex. 12A
Solution 1(a)
2,2 dimethyl propane
Solution 1(b)
2-methyl butane
Solution 1(c)
Propene
Solution 1(d)
2,2-dimethyl pentane
Solution 1(e)
Pent-2-yne
Solution 1(f)
3-methyl but-1-yne
Solution 1(g)
2,3-dimethyl pentane
Solution 1(h)
3-methyl heptane
Solution 1(i)
2-Butene
Solution 1(j)
Hept-2-yne
Solution 1(k)
5,5-dimethyl hexan-1-al
Solution 1(l)
Pentan-2-ol
Solution 1(m)
4-methyl pentan-1-oic acid
Solution 1(n)
2-bromo-2-methyl butane
Solution 1(o)
1-bromo-3-methyl butane
Solution 1(p)
Prop-1-yne
Solution 1(q)
Methanal
Solution 1(r)
1-propyne
Solution 1(s)
Propanol
Solution 1(t)
Ethanoic acid
Solution 1(u)
Ethanal
Solution 1(v)
1,2-dichloroethane
Solution 2
The structure of the following compounds are:
(a) Prop-1-ene
CH3-CH=CH2
(b) 2,3-dimethylbutane
CH3-CH(CH3)-CH(CH3)-CH3
(c) 2-methylpropane
CH3-CH(CH3)-CH3
(d) 3-hexene
CH3-CH2-CH=CH-CH2-CH3
(e) Prop-1-yne
CH3-C?CH
(f) 2-methylprop-1-ene
CH3-C(CH3)=CH2
(g) Alcohol with molecular formula C4H10O
CH3-CH2-CH2-CH2-OH
Solution 3
- (iv) CnH 2n + 1 is the formula for an alkyl group. Hence it is C5H11.
- (i) A hydrocarbon of general CnH2n is C15H30
As the formula of Alkene is CnH2n. Thus n + 2n = 72 3n = 72 n = 24. By filling value we get the molecular mass 72. - (iv) The total number of carbon chains that four carbon atoms form an alkane is 2.
- (iv) Alcohol and ether are functional isomers as they have same molecular formula but different functional groups.
- (ii) The IUPAC name of this compound is: 3-methyl hexane.
Solution 4
(a) Propane and ethane are homologues.
(b) A saturated hydrocarbon does not participate in a/an addition reaction.
(c) Succeeding members of a homologous series differ by CH2.
(d) As the molecular masses of hydrocarbons increase, their boiling points Increase and melting point increase.
(e) C25H52 and C50H102 belong to the same homologous series.
(f) CO is an organic Compound.
(g) The chemical properties of an organic compound are largely decided by the functional group and the physical properties of an organic compound are largely decided by the number of carbon atoms.
(h) CHO is the functional group of an aldehyde.
(i) The root in the IUPAC name of an organic compound depends upon the number of carbon atoms in Principal Chain.
(j) But-1-ene and but-2-ene are examples of position isomerism.
Solution 5
Chain isomerism
Chain isomerism arises due to the difference in arrangement of C atoms in the chain. For example, there are two isomers of butane, C4H10. In one of them, the carbon atoms lie in a "straight chain" whereas in the other the chain is branched.
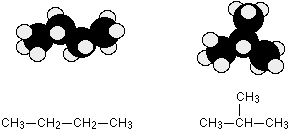
Position isomerism
It is due to the difference in position of functional groups.
For example, there are two structural isomers with the molecular formula C3H7Br. In one of them, the bromine atom is on the end of the chain, whereas in the other it is attached in the middle.
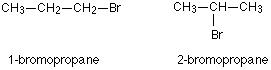
Solution 6
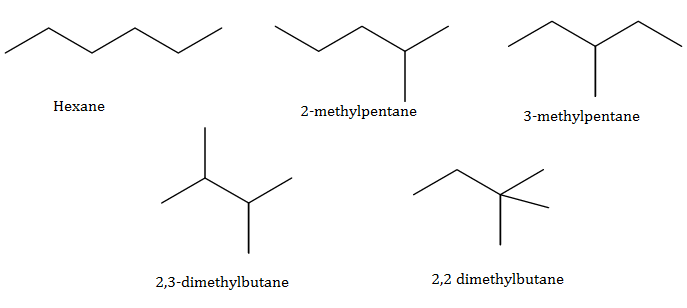
(a)Isomerism: Compounds having the same molecular formula but different structural formula are known as isomers and the phenomenon as isomerism.
Two main causes of isomerism are:
Difference in mode of linking of atoms.
Difference in the arrangement of atoms or groups in space.
(b)
(c)
CH2=CHCH2CH3 H3C-CH=CHCH3
1-butene 2-butene
Solution 7
a.
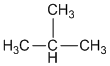
b.
![]()
c.
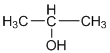
d.
![]()
e.
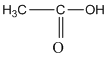
f. ![]()
Solution 8
a.
i. ![]() (Ethene)
(Ethene)
In the above structure, both carbons are bonded
with double bonds.
ii. ![]() (Ethyne)
(Ethyne)
In the above structure, both carbons are bonded
with triple bonds.
b. Addition reactions are common to both these compounds. Methane does not undergo this type of reaction because it is bounded with four hydrogen atoms, while in ethane, double bonds break and provide a site for addition.
c. Methoxymethane
Solution 9
(i) Ethane undergoes substitution reactions.
(ii) Ethene undergoes addition reactions.
Solution 10
The alkanes form an (a) electrochemical homologous series with the general formula (b) CnH2n+2. The alkanes are (c) saturated (d) hydrocarbons which generally undergo (e) substitution reactions.
Solution 11
a.
![]()
b.
![]()
c.
![]()
Solution 12
a. Ethanol
b. Ethanoic acid
c. Ethene
Solution 13
a. Propanal
b. Propanol
Solution 14
The homologous series of hydrocarbons are:
|
General Formula |
CnH2n |
CnH2n-2 |
CnH2n+2 |
|
IUPAC name of the homologous series |
Alkenes |
Alkynes |
Alkanes |
|
Characteristics bond type |
Double bond |
Triple Bond |
Single Bond |
|
IUPAC name of the first member of the series |
Ethene |
Ethyne |
Methane |
|
Type of reaction with chlorine |
Addition |
Addition |
Substitution |
Solution 15(a)
Alkenes are the (i) homologous series of (ii) unsaturated hydrocarbons. They differ from alkanes due to presence of (iii)single bonds. Alkenes mainly undergo (iv) addition reactions.
Solution 15(b)
The organic compound which undergoes substitution reaction is C2H6.
Solution 15(c)
(c) Structural formulae of isomers ofButane are:
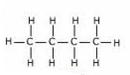
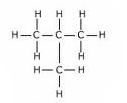
Butane2-methyl propane
Solution 16
- Ethane
- Propanol
- Ethyne
Organic Chemistry Exercise Ex. 12B
Solution 1
Sources of alkane:
The principal sources of alkanes are Natural gas and petroleum.
Solution 2
Methane is a primary constituent of natural gas. It absorbs outgoing heat radiation from the earth, and thus contributes to the green house effect and so it is considered as a green house gas.
Solution 3
The general formula of alkane is :
CnH 2n+2
Solution 4
(a)The structures of isomers of butane are:
(i)
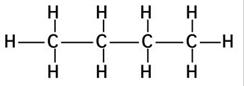
Common name:- n-Butane
IUPAC name:- Butane
(ii)
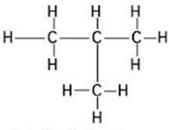
Common name:-iso butane
IUPAC name:- 2-methyl propane
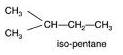
(b) The structures of isomers of Pentane are:
(i)
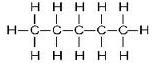
Common name: n-pentane
IUPAC name:- Pentane
(ii)
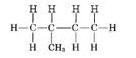
Common name:- iso pentane
IUPAC name:- 2-methyl butane
(iii)
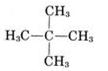
Common name- neo pentane
IUPAC name:- 2,2-dimethyl propane
Solution 5
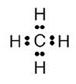
For methane:
(a) Molecular formula is CH4
(b) Electron dot formula
(c) Structural formula
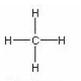
For ethane:
(a) Molecular formula is :- C2H6
(b) Electron dot formula:
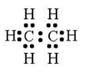
(a) Structural Formula:
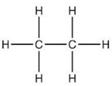
Solution 6
(a) Laboratory preparation of methane:
When the mixture of sodium ethanoate and soda lime is taken in a hard glass test tube and heated, the gas evolved is methane. It is collected by downward displacement of water.
CH3COONa+NaOH![]() Na2CO3+CH4
Na2CO3+CH4
(b) Laboratory preparation of ethane:
When the mixture of sodium propionate and soda lime is taken in the boiling tube and heated the ethane gas is evolved. It is also collected by downward displacement of water.
C2H5COONa+NaOH![]() Na2CO3+C2H6
Na2CO3+C2H6
Solution 7
When methyl iodide is reduced by nascent hydrogen at ordinary room temperature then methane is formed.
CH3I+2[H] ![]() CH4+HI
CH4+HI
When bromoethane is reduced by nascent hydrogen at ordinary room temperature then ethane is produced.
C2H5Br+2[H] ![]() C2H6+HBr
C2H6+HBr
Solution 8
A reaction in which one atom of a molecule is replaced by another atom (or group of atoms)is called a substitution reaction.
When ethane reacts with chlorine
C2H6 +Cl2 ![]() C2H5Cl
+ HCl
C2H5Cl
+ HCl
Chloroethane
C2H5Cl + Cl2![]() C2H4Cl2+HCl
C2H4Cl2+HCl
Dichloroethane
C2H4Cl2 +Cl2 ![]() C2H3Cl3+HCl
C2H3Cl3+HCl
Trichloroethane
C2H3Cl3 + Cl2 ![]() C2H2Cl4
+ HCl
C2H2Cl4
+ HCl
Tetrachloroethane
C2H2Cl4 +Cl2 ![]() C2HCl5
+HCl
C2HCl5
+HCl
Pentachloroethane
C2HCl5 +Cl2 ![]() C2Cl6
+ HCl
C2Cl6
+ HCl
Hexachloroethane
Solution 9
(a) Sufficient air: When methane burns in sufficient air, then carbon dioxide and water vapors are formed.
CH4 + 2O2 ![]() CO2+2H2O
CO2+2H2O
(b) Insufficient air: When methane burns in insufficient air , then carbon monoxide and water is formed.
2CH4 + 3O2 ![]() 2CO + 4H2O
2CO + 4H2O
Solution 10
(a)
(i) When methane reacts with chlorine in the presence of sunlight or UV light, it undergoes substitution reaction to form Tetrachloromethane.
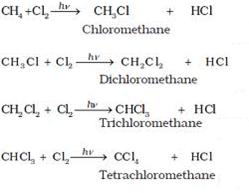
(ii) When it reacts with bromine it forms Tetrabromomethane
CH4 + Br2![]() CH3Br + HCl
CH3Br + HCl
CH3Br + Br2![]() CH2Br2 + HCl
CH2Br2 + HCl
Dibromomethane
CH2Br2 + Br2![]() CHBr3 + HCl
CHBr3 + HCl
Tribromo methane
CHBr3 + Br2![]() CBr4 + HCl
CBr4 + HCl
Tetrabromomethane
(b)
(i) When ethane reacts with chlorine it forms hexachoroethane.
C2H6 +Cl2 ![]() C2H5Cl
+ HCl
C2H5Cl
+ HCl
Chloroethane
C2H5Cl + Cl2![]() C2H4Cl2+HCl
C2H4Cl2+HCl
Dichloroethane
C2H4Cl2 +Cl2 ![]() C2H3Cl3+HCl
C2H3Cl3+HCl
Trichloroethane
C2H3Cl3 + Cl2 ![]() C2H2Cl4
+ HCl
C2H2Cl4
+ HCl
Tetrachloroethane
C2H2Cl4 +Cl2 ![]() C2HCl5
+HCl
C2HCl5
+HCl
Pentachloroethane
C2HCl5 +Cl2 ![]() C2Cl6
+ HCl
C2Cl6
+ HCl
Hexachloroethane
(ii) When ethane reacts with bromine it forms Hexabromoethane
C2H6 +Br2 ![]() C2H5Br
+ HBr
C2H5Br
+ HBr
Bromoethane
C2H5Br + Br2![]() C2H4Br2+HBr
C2H4Br2+HBr
Dibromoethane
C2H4Br2 +Br2 ![]() C2H3Br3+HBr
C2H3Br3+HBr
Tribromoethane
C2H3Br3 + Br2 ![]() C2H2Br4
+ HBr
C2H2Br4
+ HBr
Tetrabromoethane
C2H2Br4 +Br2 ![]() C2HBr5
+HBr
C2HBr5
+HBr
Pentabromoethane
C2HBr5 +Br2 ![]() C2Br6
+ HBr
C2Br6
+ HBr
HexaBromoethane
Solution 11
(a) Ethane is prepared from sodium propionate.
C2H5COONa+NaOH![]() Na2CO3+C2H6
Na2CO3+C2H6
(b) Methane is prepared from methyl iodide.
CH3I+2[H] ![]() CH4+HI
CH4+HI
(c) Ethane is prepared from ethyl bromide.
C2H5Br+2[H] ![]() C2H6+HBr
C2H6+HBr
Solution 12
(i) ![]()
(ii) ![]()
Solution 13
(a) Methane into chloroform
CH4+Cl2![]() CH3Cl+HCl
CH3Cl+HCl
CH3Cl+Cl2![]() CH2Cl2+HCl
CH2Cl2+HCl
CH2Cl2+Cl2![]() CHCl3+HCl
CHCl3+HCl
(b) Sodium acetate into methane
CH3COONa+NaOH![]() Na2CO3+CH4
Na2CO3+CH4
(c) Methyl iodide into ethane
2CH3I +2Na![]() CH3-CH3+2NaI
CH3-CH3+2NaI
(d) Methane to methyl alcohol
Solution 14
(a) Methane: Three uses of methane are:
(i) Methane is a source of carbon monoxide and hydrogen
(ii) It is used in the preparation of ethyne, methanal, chloromethane, carbon tetrachloride.
(iii) It is employed as a domestic fuel.
(b) Ethane:
Three uses of ethane are:
(i) It is used in the preparation of ethene, ethanol, and ethanol.
(ii) It forms ethyl chloride, which is used to make tetraethyllead.
(iii) It is also a good fuel.
Solution 15
(a) When a mixture of ethane and oxygen is compressed to about 120atm pressure and passed over copper tubes at 475K, ethyl alcohol is formed.
2C2H6 +O2![]() 2C2H5OH
2C2H5OH
(b) When mixture of ethane and oxygen is passed through heated molybdenum oxide, the mixture is oxidized to Acetaldehyde.
C2H6 +O2 ![]() CH3CHO+H2O
CH3CHO+H2O
(c) Ethanol formed from ethane gets oxidized to acetic acid.
2C2H6 +O2![]() 2C2H5OH
2C2H5OH
C2H5OH + O2 ![]() CH3COOH+H2O
CH3COOH+H2O
Solution 16
Ethane can be oxidized as follows:
When a mixture of ethane and oxygen in the ratio 9:1 by volume is compressed to about 120 atm pressure and passed over copper tubes at 475K, ethyl alcohol is formed.
2C2H6 + O2 ![]() 2C2H5OH
2C2H5OH
When a mixture of ethane and oxygen is passed through heated MoO, the mixture is oxidized to ethanal.
C2H6+ O2 ![]() CH3CHO + H2O
CH3CHO + H2O
When a manganese based catalyst is used 100oC, ethane can be oxidized to ethanoic acid.
2C2H6 + 3O2 ![]() 2CH3COOH + 2H2O
2CH3COOH + 2H2O
Organic Chemistry Exercise Ex. 12C
Solution 1
(a) The molecular formula of ethene is C2H4
(b) Electron dot formula of ethene is:
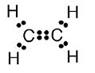
(c) Structural formula of ethene:
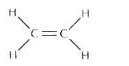
Solution 2
(a) n signifies the number of carbon atoms and 2n signifies the number of hydrogen atoms.
(b) The name of alkene when n=4 is Butene.
(c) The molecular formula of alkene when n=4 is C4H8.
(d) The molecular formula of alkene when there are 10 H atom in it C5H10.
(e) The structural formula of the third member of alkene is
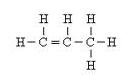
(f) Lower homologus of alkene which contain four carbons is C3H6.
Higher homologus of alkene which contain four carbons is C5H10.
Solution 3(a)
|
Ethane |
Ethene |
|
|
|
|
It has carbon -carbon single bond.
|
It has carbon-carbon double bond
|
|
It is saturated.
|
It is unsaturated
|
|
Alkanes undergo substitution reaction. |
Alkenes undergo addition reaction. |
Solution 3(b)
Isomers of butene are but-2-ene and but-1-ene and their structures are as follows:
Solution 4
Balanced Equation of ethylene:
CH3-CH2OH + H2SO4
![]() CH3-CH2HSO4+H2O
CH3-CH2HSO4+H2O
CH3-CH2HSO4![]() CH2=CH2
CH2=CH2
The gas is collected by downward displacement of water.
Solution 5
(a) Dehydrohalogenation reaction:
C2H5Cl + KOH(alc.and hot) ![]() C2H4
+ KCl + H2O
C2H4
+ KCl + H2O
Ethene
(b) Dehydration reaction:
C2H5OH ![]() C2H4+H2O
C2H4+H2O
Ethene
Solution 6(a)
Chlorine and bromine are added to the double bond of ethene to form saturated ethylene chloride and ethylene bromide respectively.
CH2 = CH2 + Cl2 ![]() CH2(Cl)-CH2(Cl)
CH2(Cl)-CH2(Cl)
1,2-dichloro ethane
CH2 = CH2 + Br2 ![]() CH2(Br)-CH2(Br)
CH2(Br)-CH2(Br)
1,2-dibromo ethane
Solution 6(b)
When ethene and hydrogen are passed over finely divided catalyst such as platinum or palladium at ordinary temperature or nickel at 200o C, the two atom of hydrogen molecule are added to the unsaturated molecule, which thus becomes a saturated one.
C2H4 +H2 ![]() C2H6
C2H6
Solution 7
Conversion of ethanol to ethene by using
(a) Solid dehydrating agent:
![]()
(b) Hot conc. H2SO4:
![]()
Solution 8
(a) Physical state: Ethene is a colourless and inflammable gas.
(b) Odour: It has faint sweetish odour.
(c) Density as compared to air: It has density less than one hence it is lighter than air.
(d) Solubility: It is sparingly soluble in water but highly soluble in organic solvents like alcohol, ether and chloroform.
Solution 9
(a) Ethyl bromide into ethane
Ethyl bromide in the presence of hot alcoholic KOH undergoes dehydrohalogenation reaction to give ethane.
C2H5Br + KOH → C2H4 + KBr + H2O
Alc. Hot & conc. Ethene
(b) Ethene into 1,2-dibromoethane
Ethene reacts with bromine at room temperature in presence of CCl4 to form saturated 1, 2-Dibromoethane.
This reaction is an addition reaction of alkene to saturated dibromide in the presence of CCl4.
CH2 = CH2 + Br2 + CCl4 → CH2(Br)-CH2(Br)
(c)Ethene into ethane:
Ethene is converted into ethane by hydrogenation in the presence of nickel catalyst.
Ethene Ethane
Solution 10
(a) C2H4+3O2 ![]() 2CO2 +2H2O + heat
2CO2 +2H2O + heat
(b) CH2=CH2+Cl2 ![]() CH2(Cl)-CH2(Cl)
CH2(Cl)-CH2(Cl)
(c) CH2=CH2 + HCl ![]() CH3-CH2-Cl
CH3-CH2-Cl
(d) C2H4 +H2 ![]() C2H6
C2H6
Solution 11
(a) CH4![]() CH3Cl
CH3Cl![]() CH2Cl2
CH2Cl2![]() CHCl3
CHCl3![]() CCl4
CCl4
A= monochloromethane
B= dichloromethane
C=Trichloromethane
D=Tetrachloromethane
(b) C2H2![]() C2H4
C2H4![]() C2H6
C2H6![]() C2H5Br
C2H5Br![]() C2H4Br
C2H4Br
A= Ethene
B=ethane
C=bromoethane
D=dibromoethane
(c) C2H4 +H2![]() C2H6
C2H6
B= hydrogen
Solution 12
(a) C2H4 +Cl2 ![]() CH2(Cl)-CH2(Cl)
CH2(Cl)-CH2(Cl)
1,2- dichloro ethane
(b)
Ethane
(c) CH2=CH2 ![]() CH2(OH)-CH2(OH)
CH2(OH)-CH2(OH)
1,2- Ethanediol
(d) CH2=CH2+HBr ![]() CH3-CH2Cl
CH3-CH2Cl
chloroethane
Solution 13
When ethylene is passed through alkaline KMnO4 solution 1, 2-Ethanediol is formed. The Purple color of KMnO4 decolorizes.
CH2=CH2+H-O-H +[O] ![]() CH2(OH)-CH2(OH)
CH2(OH)-CH2(OH)
Cold alkaline
KMnO4 solution
Solution 14
Three compounds formed by ethylene are:
Polythene
Ethanol
Epoxyethane
Uses of above compounds:
Polythene is used as carry bags.
Ethanol is used as a starting material for other products, mainly cosmetics and toiletry preparation.
Epoxyethane is used in the manufacture of detergents.
Organic Chemistry Exercise Ex. 12D
Solution 1
Natural gas and Petroleum are sources for alkynes.
The general formula of alkynes are:
CnH2n-2
Solution 2
Butyne is an example, its isomers are:
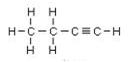
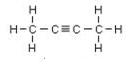
IUPAC name: But-2-yne But-1-yne
Solution 3
(a) Diagram of acetylene preparation:
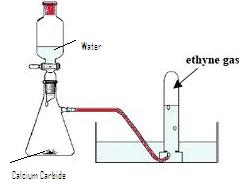
(b) CaC2 +2H2O ![]() Ca(OH)2 +C2H2
Ca(OH)2 +C2H2
(c) The pure dry gas is collected by downward displacement of water, since it is insoluble in water.
Solution 4
When 1,2 -dibromoethane is boiled with alcoholic potassium hydroxide ,ethyne is formed.
![]()
Solution 5
(a) The hydrocarbon which is tetrahedral is Methane.
(b) The hydrocarbon which is planar molecule is ethene.
(c) The hydrocarbon which is a linear molecule is Ethyne.
(d) The hydrocarbon which forms a red precipitate with ammoniacal solution of copper chloride is acetylene.
(e) Alkanes are also called as paraffin.
(f) Alkenes are also called olefin.
(g) Calcium carbide
Solution 6
The following compounds can be classified as:
C3H4:- Alkynes
C3H8:- Alkanes
C5H8:- Alkynes
C3H6:- Alkenes
Solution 7
Chemical test to distinguish :
(b) Ethane and ethene:
|
S.No. |
Test |
Ethane |
Ethene |
|
1. |
On adding a few drops of bromine solution in carbon tetrachloride to the hydrocarbon |
No change is observed |
The reddish brown colour gets decolorized |
|
2. |
On adding a few drops of alkaline potassium permanganate (purple colour) to the hydrocarbon |
No change is observed |
The purple colour fades. |
(c) Ethene and ethyne:
|
S.No. |
Test |
Ethene |
Ethyne |
|
1. |
On adding a few drops of ammonical cuprous chloride to the hydrocarbon |
No change is observed |
Red precipitate of copper acetylide is formed |
|
2. |
On adding ammonical silver nitrate |
No observation |
White precipitate of silver acetylide is formed. |
Solution 8
(a) HC≡CH
(b) Brown colour of CCl4 disappeared due to formation of addition product, i.e. 1, 2-dibromo ethane.
Solution 9
- CH2 =CH2 + H2
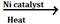 CH3 -CH3
CH3 -CH3 - CH2 =CH2 + H2O ⟶ CH3 -CH2-OH
- CH ≡CH + H2
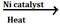 CH2 =CH2
CH2 =CH2
Solution 10
(a) Ethyne in an inert solvent of carbon tetrachloride adds chlorine to change into 1,2-dichloro ethene with carbon-carbon double bond, and then to an 1,1,2,2-tetrachloro ethane with carbon-carbon single bond.
C2H2![]() C2H2Cl2
C2H2Cl2![]() C2H2Cl4
C2H2Cl4
1,2-dichloro ethene1,1,2,2 -tetrachloro ethane
(b) Ethyne in an inert solvent of carbon tetrachloride adds bromine to change into 1,2-dibromo ethene and then to 1,1,2,2 -tetrabromo ethane .
C2H2![]() C2H2Br2
C2H2Br2![]() C2H2Br4
C2H2Br4
(c) Iodine reacts slowly in the presence of alcohol to form di-iodo ethene
CH![]() CH +I2
CH +I2 ![]() ICH=CHI
ICH=CHI
1,2-di-iodoethene
(d) In the presence of nickel, platinum or palladium ethyne change to ethene and then to ethane.
CH![]() CH
CH ![]() CH2=CH2
CH2=CH2![]() CH3-CH3
CH3-CH3
(e) ![]()
Solution 11
Substitution reactions are characteristic reactions of alkanes.
Solution 12
(a) i. 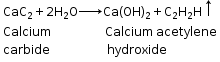
ii. 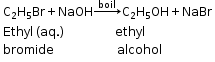
(b) When bromine in carbon tetrachloride is added to ethyne, the orange colour of the bromine disappears due to the formation of the colourless ethylene bromide.
(c) Water reacts with ethene to form ethanol.
CH2=CH2 +H2O![]() C2H5OH
C2H5OH
Solution 13
(a) Ethyne is a highly reactive compound than ethene because of the presence of a triple bond between its two carbon atoms.
(b) Ethene is a highly reactive compound than ethane because of the presence of a double bond between its two carbon atoms.
(c) Hydrocarbons such as alkanes undergo combustion reactions with oxygen to produce carbon dioxide and water vapour. Alkanes are flammable which makes them excellent fuels.
Methane for example is the principal component of natural gas.
CH4 + 2O2 → CO2 + 2H2O
Solution 14
a.
i. ![]()
ii. ![]()
b.
i. ![]()
ii.
![]()
Solution 15
(a)Preparation of carbon tetrachloride from methane:
CH4+Cl2 ![]() CH3Cl +HCl
CH3Cl +HCl
CH3Cl + Cl2 ![]() CH2Cl2 +HCl
CH2Cl2 +HCl
CH2Cl2 +Cl2 ![]() CHCl3 +HCl
CHCl3 +HCl
CHCl3 + Cl2 ![]() CCl4 +HCl
CCl4 +HCl
(b)Structural formula of ethyne:
![]()
(c)Alkynes contain triple bond where as alkenes contain double bond.
Organic Chemistry Exercise Ex. 12E
Solution 1
(a) Alcohols are the hydroxyl derivatives of alkanes and are formed by replacing one or more hydrogen atoms of the alkane with -OH group.
Sources of alcohols:
Methanol is obtained from destructive distillation of wood while ethanol is obtained from fermentation of sugar.
(b) General formula of monohydric alcohol: CnH2nOH
Solution 2
(a) First member of alcohol is metanol
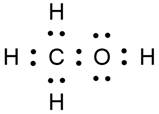
(b) Abbreviated formula of third member of alcohol is propanol (CH3CH2CH2OH) is C3H8O.
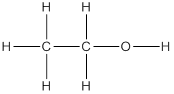
(c) Second member of alcohol family is ethanol and its structure is as follows:
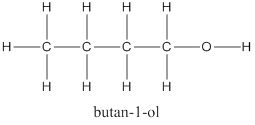
(d) Alcohol with 4 carbon atoms is Butan-1-ol and its structure is as follows:
Solution 3
The method of preparation of ethanol:
(a) By hydrolysis of ethene :
When concentrated sulphuric acid is added to ethene at a temperature of 80° C and pressure of 30 atm, ethyl hydrogen sulphate is formed. Ethyl hydrogen sulphate on hydrolysis with boiling water gives ethanol.
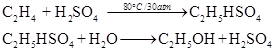
(b) By hydrolysis of ethyl bromide :
Alcohols are prepared by the hydrolysis of an alkyl halide with a hot dilute alkali.
![]()
Solution 4
The second member of homologous series of alcohol is 'Ethanol'.
Ethanol can be prepared by the hydrolysis of a haloalkane ethyl chloride with a hot dilute alkali.
The chemical equation for this reaction is as follows:
![]()
Solution 5
(a)The melting and boiling point of the successive members of the homologous series of alcohols increase with the increase in molecular mass.
(b)When ethanol reacts with acetic acid ethyl acetate is formed.
C2H5OH + CH3COOH ![]() CH3COOC2H5 + H2O
CH3COOC2H5 + H2O
(c) This reaction is known as esterification reaction.
Solution 6
(a) CH![]() CH +H2
CH +H2 ![]() CH2=CH2 +H2
CH2=CH2 +H2 ![]() CH3-CH3
CH3-CH3
(b) C2H4 + Cl2 ![]() CH2(Cl)-CH2(Cl)
CH2(Cl)-CH2(Cl)
(c) C2H4 + HCl ![]() CH3-CH2Cl
CH3-CH2Cl
(d) CaC2 + 2H2O ![]() C2H2+Ca(OH)2
C2H2+Ca(OH)2
(e) C2H2 + Br2 ![]() H(Br)C=C(Br)H
H(Br)C=C(Br)H
(f) C2H5OH ![]() CH3CHO
CH3CHO
Solution 7
Ethanol affects that part of the brain which controls our muscular movements and then gives temporary relief from tiredness. But it damages the liver and kidney too.
Solution 8
(a) Absolute alcohol: Absolute alcohol may be obtained by distilling moist alcohol with benzene. The mixture of water and benzene distills off and anhydrous alcohol is left behind.
(b) Spurious alcohol: It is made by improper distillation. It contains large portions of methanol in a mixture of alcohols.
(c) Methylated spirit: Methylated spirit or denatured alcohol is ethyl alcohol with 5%methyl alcohol, a coloured dye and some pyridine.
Solution 9
(a) Sodium reacting with ethyl alcohol:
2C2H5OH + 2Na ![]() 2C2H5ONa + H2
2C2H5ONa + H2
When sodium reacts with ethyl alcohol hydrogen is evolved with formation of sodium ethoxide.
(b) Ethanol oxidized by K2Cr2O7:
C2H5OH![]() CH3CHO+H2O
CH3CHO+H2O![]() CH3COOH
CH3COOH
Alcohols gets oxidized and get converted into ethanal and then into acetic acid.
Solution 10
|
S No |
Formula |
Common Name |
IUPAC |
|
1 |
C3H6 |
Propylene |
Propene |
|
2 |
C2H4 |
Ethylene |
Ethene |
|
3 |
C2H2 |
Acetylene |
Ethyne |
|
4 |
CH3OH |
Methyl alcohol |
Methanol |
|
5 |
C2H5OH |
Ethyl alcohol |
Ethanol |
Solution 11
C2H5OH![]() CH3CHO+H2O
CH3CHO+H2O![]() CH3COOH
CH3COOH
The oxidizing agents that can be used are potassium dichromate and potassium permanganate.
Solution 12
- Used for illuminating country houses : Ethyne
- Used for making a household plastic material: ethyne
- Called 'wood spirit' : Methanol
- Poisonous: Methanol
- Consumed as a drink: Ethanol
- The organic compound which is used in thermometer is ethanol.
- Ethanol
- Ethyl alcohol
Solution 13
(a) The conversion of ethanol into ethene is an example ofDehydration.
(b) Converting ethanol into ethene requires the use of Conc. H2SO4.
(c) The conversion of ethene into ethane is an example of hydrogenation.
(d) The catalyst used in the conversion of ethene into ethane is commonly nickel.
Solution 14
(a)Ethane from sodium propionate
C2H5COONa + NaOH ![]() Na2CO3 + C2H6
Na2CO3 + C2H6
(b)Ethene from iodoethane
C2H5 I +KOH(alcoholic) ![]() C2H4 +KI + H2O
C2H4 +KI + H2O
(c)Ethyne from calcium carbide
CaC2 +2H2O ![]() Ca(OH)2 + C2H2
Ca(OH)2 + C2H2
(d)Methanolfrom iodoethane
CH3I + NaOH ![]() CH3OH + NaI
CH3OH + NaI
Solution 15
(i) C2H5COONa +NaOH![]() Na2CO3 + C2H6
Na2CO3 + C2H6
(ii) CH3I +2[H] ![]() CH4 +HI
CH4 +HI
(iii) C2H5Br + KOH ![]() C2H4 +KBr +H2O
C2H4 +KBr +H2O
(iv) CO + 2H2 ![]() CH3OH
CH3OH
(v) CaC2 +2H2O ![]() Ca(OH)2 +C2H2
Ca(OH)2 +C2H2
Solution 16
(a) Calcium carbide and water:
CaC2 + 2H2O ![]() Ca(OH)2 + C2H2
Ca(OH)2 + C2H2
(b) Ethene and water:
CH2=CH2 +H2O ![]() C2H5OH
C2H5OH
(c) Bromoethane and aqueous solution of sodium hydroxide
C2H5Br +NaOH ![]() C2H5OH +NaBr
C2H5OH +NaBr
Organic Chemistry Exercise Ex. 12F
Solution 1
An organic compound containing the carboxyl group(COOH) is known as carboxylic acid.
The general formula: CnH2n+1COOH
Solution 2
(a) First three members of carboxylic acids are:
Methanoic acid
Ethanoic acid
Propanoic acid
(b) Three compounds that can be oxidized directly or in stages to produce acetic acid are:
Ethanol
Acetylene
Ethanal
Solution 3
(a) The structural formula of acetic acid is as follows:
(b) IUPAC name of acetic acid is Ethanoic acid.
(c) Glacial acetic acid is the anhydrous (water-free) acetic acid.
Solution 4
Vinegar commonly called Sirka is a dilute solution of acetic acid. The presence of colouring matter gives it a greyish colour while the presence of some other organic acids and organic compounds impart it the usual taste and flavour.
Solution 5
(a) Ethanol
(b) Acetic acid
(c) Propanoic acid
Solution 6
(a)It is prepared in the lab by the oxidation of ethanol with acidified potassium dichromate.
C2H5OH![]() CH3CHO
CH3CHO ![]() CH3COOH
CH3COOH
(b)Acetylene is first converted to acetaldehyde by passing through 40% H2SO4 at 60°C in the presence of 1% HgSO4.
The acetaldehyde is then oxidised to acetic acid in the presence of catalyst manganous acetate at 70°C.
C2H2 + H2O ![]() CH3CHO
CH3CHO
CH3CHO + O2 ![]() 2CH3COOH
2CH3COOH
Solution 7
(a) When acetic acid reacts with litmus it turns blue litmus red.
(b) When acetic acid reacts with metals hydrogen is evolved.
2CH3COOH + Zn ![]() (CH3COO)2Zn
+ H2
(CH3COO)2Zn
+ H2
(c) When acetic acid reacts with alkalies it forms salt
CH3COOH + NaOH ![]() CH3COONa
+ H2O
CH3COONa
+ H2O
(d) Acetic acid reacts with alcohols forming esters
CH3COOH + C2H5OH
![]() CH3COOC2H5 + H2O
CH3COOC2H5 + H2O
Solution 8
(a) 2CH3COOH + Zn![]() (CH3COO)2Zn + H2
(CH3COO)2Zn + H2
(b) CH3COOH + NaOH ![]() CH3COONa + H2O
CH3COONa + H2O
(c) 2CH3COOH + Na2CO3 ![]() 2CH3COONa +H2O+ CO2
2CH3COONa +H2O+ CO2
(d) CH3COOH + NaHCO3 ![]() CH3COONa + H2O +CO2
CH3COONa + H2O +CO2
Solution 9
(a) When acetic acid is added to sodium bicarbonate, carbondioxide is liberated.
CH3COOH + NaHCO3 ![]() CH3COONa + H2O +CO2
CH3COONa + H2O +CO2
(b) When acetic acid is added to ethyl alcohol in presence of sulphuric acid ester (ethyl acetate) is formed.
CH3COOH + C2H5OH ![]() CH3COOC2H5 + H2O
CH3COOC2H5 + H2O
(c) When acetic acid is added to neutral FeCl3, wine red color is produced.
Solution 10
(a) When acetic acid and ethanol react it results in the formation of ethyl acetate.
(b) Lithum aluminium hydride(LiAlH4) is used to convert acetic acid to ethanol.
(c) Phosphorous pentoxide(P2O5) is heated along with acetic acid to form acetic anhydride.
Organic Chemistry Exercise Misc. Ex.
Solution 1 (a)
Correct option: (ii) - They can undergo addition as well as substitution reaction.
Alkanes can only undergo substitution reaction.
Solution 1 (b)
Correct option: (ii) - Ethanol
Ethanol is the organic compound obtained as the end product of the fermentation of sugar solution
Solution 1 (c)
Odd one is C5H10.
From the given molecular formulae, C3H8, C2H6, CH4 belong to alkane homologous series with general formula CnH2n+2.
But, C5H10 belong to alkene homologous series with general formula CnH2n.
Solution 2
(a) The laboratory preparation of methane from sodium acetate:
![]()
(b) The reaction of one mole of ethene with one mole of chlorine gas
![]()
(c) The preparation of ethyne from 1, 2-bibromoethane.
When 1, 2-dibromoethane (ethylene dibromide) is boiled with alcoholic potassium hydroxide, ethyne is formed. The halogen bromine gets detached from the reactant to give potassium bromide. Alcoholic is used for dehydrohalogenation.
![]()
Solution 3
(a) Ethyl chloride to Ethyl alcohol
Ethyl chloride is converted to Ethyl alcohol by the substitution Reaction where it is treated with aq. KOH or aq. NaOH.
The balanced equation is as follows:
![]()
(b) Ethyl chloride to Ethene
![]()
(c) Ethene to Ethyl alcohol
When concentrated sulphuric acid is added to ethene at a temperature of 80° C and pressure of 30 atm, ethyl hydrogen sulphate is formed. Ethyl hydrogen sulphate on hydrolysis with boiling water gives ethanol.
![]()
(d) Ethyl alcohol to Ethene
![]()
Solution 4
(a) Isomerism:
Compounds having the same molecular formula but different structural formula are known as isomers and the phenomenon as isomerism.
(b) The IUPAC name of the isomer C4H10 which has a branched chain:
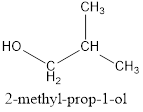
Solution 5
(a) X = Ethanol, C2H5OH
Y = Acetic acid, CH3COOH
Z = Conc. H2SO4
(b)
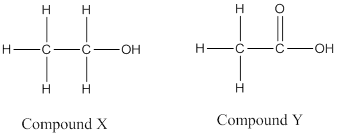
(c) Compound P is an ester named ethyl acetate.
(d) Esterification
(e) ![]()
Solution 6
(a) Equation of ethyl bromide with aqueous NaOH
CH3CH2Br(aq) + NaOH (aq) → CH3CH2OH(aq) + NaBr(aq)
(b) Equation of ethyl bromide with alcoholic NaOH
CH3CH2Br(aq) + NaOH (alc.) → CH2CH2 + HBr
Solution 2010
a. iii. Ethyne
b. i. Formic acid
c. i. Methanol
d. i. 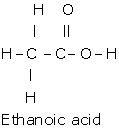
ii 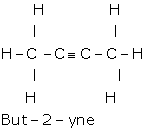
e.
i. ![]()
ii. Addition reaction
iii. Bromine solution gets decolourised
iv. Ethanol
v. By heating it (ethanol) with concentrated sulphuric acid at 170°C
Solution 2011
a. iv. Carboxyl - COOH
b. iii. An addition reaction
c. iii Six
d.
i. Nickel
ii. Acetic acid
iii. Esterification
iv.
![]()
v. Ethanol
e.
i. ![]()
ii. ![]()
iii. ![]()
iv. CaC2 + 2H2O → Ca(OH)2 + C2H2
v. 2C2H5OH + 2Na → 2C2H5ONa + H2
Solution 2012
a. i.
![]()
ii.
![]()
iii. ![]()
iv.
![]()
v.
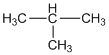
b. i. Ethyne
ii. Acetic acid
iii. Ethene
iv Ethanol
c. i. Pure acetic acid is called glacial acid because it forms an ice-like solid when cooled.
ii. ![]()
Solution 2013
a.
i. Ethene gas decolourises the purple colour of KMnO4, whereas ethane does not decolourise KMnO4 solution.
ii. They can undergo both substitution as well as addition reactions.
b.
i. Isomer of n-butane: Isobutane
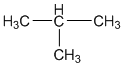
ii. 2-propanol
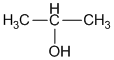
Solution 2014
a. (iv) ethyne
b. Ketones
c.
![]()
d. Ethene gas decolourises the purple colour of KMnO4, whereas ethane does not decolourise KMnO4 solution.
e.
i. Ethanol:
![]()
ii. 1-propanal:
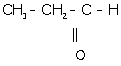
iii. Ethanoic acid:
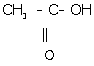
iv. 1, 2-dichloroethane:
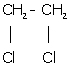
f.
The balanced chemical equation for the preparation
of ethanol from monochloroethane and aqueous
sodium hydroxide:
C2H5-Cl + NaOH (aq.) ![]() C2H5OH + NaCl
C2H5OH + NaCl
Solution 2015
(a)
(i) Ethanoic acid to ethyl ethanoate
CH3COOH + C2H5OH ![]() CH3COOC2H5 + H2O
CH3COOC2H5 + H2O
(ii) Calcium carbide to ethylene
CaC2 + 2 H2O → Ca(OH)2 + CH º CH
(iii) Sodium ethanoate to methane
CH3COONa + NaOH ![]() Na2CO3 + CH4
Na2CO3 + CH4
(b)
(i) Dimethyl ether
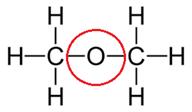
(ii) Propanone
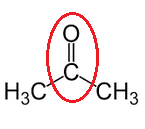
(c) Name the following:
(i) Hydrogenation
(ii) Methane
(iii) Esterification
(iv) Catenation
(v) Dehydrohalogenation
(d) Correct option: (D)-Methane
Methane produces greenhouse effect.