Class 10 NCERT Solutions Chemistry Chapter 3 - Metals and Non-metals
Metals and Non-metals Exercise 40
Solution 1
(ii) Metal that can be easily cut with a knife
(iii) Metal that is the best conductor of heat
(iv) Metals that are poor conductors of heat
Concept insight: For answering this question, recall the metals which have the properties stated in the question. If only name of the metal is asked, write the name only.
Solution 2
Ductile: Substances that can be drawn into thin wires are called ductile. For example -most of the metals like copper and aluminium are ductile.
Concept insight: For answering this question, write the complete definition of melleable and ductile. Remember the key words - malleable related to thin sheets and ductile related to wires.
Metals and Non-metals Exercise 46
Solution 1
Concept insight: The key to this answer is that sodium is a very reactive metal.
Solution 2
(i) 3 Fe (s) + 4 H2O (g) ![]() Fe3O4 (s)+ 4 H2( g)
Fe3O4 (s)+ 4 H2( g)
(ii) Ca(s) + 2 H2O(l) ![]() Ca(OH)2 (aq) + H2 (g) + Heat
Ca(OH)2 (aq) + H2 (g) + Heat
(iii) 2K (s) + 2 H2O(l) ![]() 2 KOH (aq) + H2 (g) + Heat
2 KOH (aq) + H2 (g) + Heat
Concept insight: For answering this question , write the balanced chemical equations.
Solution 3
(ii) When metal B is added to a solution of copper (II) sulphate, a displacement reaction occurs due to which blue colour of copper sulphate solution fades and a red brown coating of copper forms on B.
B + CuSO4
(iii) The arrangement of the metals in the order of decreasing reactivity is:
B > A > C > D
Concept insight: The key to this answer is displacement reaction. A displacement reaction takes place when a more reactive metal is able to displace a less reactive metal from its salt solution.
A + FeSO4
A + CuSO4
B + FeSO4
B + ZnSO4
C + FeSO4
C + CuSO4
C + ZnSO4
C + AgNO3
D + FeSO4 / CuSO4 / ZnSO4 / AgNO3
Solution 4
When iron reacts with dilute H2SO4, iron (II) sulphate with the evolution of hydrogen gas is formed.
Fe (s) + H2SO4 (aq)
Concept insight: For answering this question, you need to recall the reaction between metals and dilute acids.
Solution 5
Zinc is more reactive than iron. Therefore, if zinc is added to a solution of iron (II) sulphate, then it would displace iron from the solution and a colourless solution of zinc sulphate is formed.
Zn (s) + FeSO4 (aq) ![]() ZnSO4 (aq) + Fe (s)
ZnSO4 (aq) + Fe (s)
Concept insight: For answering this question, recall the reaction between zinc is added to a solution of iron (II) sulphate.
Metals and Non-metals Exercise 49
Solution 1
(i) The representation of elements with valence electrons as dots around the elements is referred to as electron-dot structure for elements.
(iii) The ions present in Na2O are Na+ and O2? ions and in MgO are Mg2+ and O2? ions.
Concept insight: For answering this question, remember that metals lose electrons and form positively charged cations. Non- metals gain electrons and form negatively charged anions.
Solution 2
Concept insight: The key to this answer is in the type of bonding that exists in ionic compounds.
Metals and Non-metals Exercise 53
Solution 1
(ii) Ore: Minerals from which metals can be extracted conveniently and profitably are known as ores.
(iii) Gangue: The unwanted impurities (sand, silt, soil, gravel, etc.) present in the ore are called gangue.
Concept insight: For answering this question, write the proper definition of all the three terms.
Solution 2
Concept insight: The key to this answer is that what type of metals will exist in free state.
Solution 3
For example, zinc oxide is reduced to metallic zinc by heating with carbon.
Concept insight: The key to this answer is that the conversion of metal oxide to metal is a reduction of metal oxide.
Metals and Non-metals Exercise 55
Solution 1
A more reactive metal displaces a less reactive metal from its oxide. Out of zinc, magnesium and copper, magnesium is the most reactive, zinc is less reactive and copper is the least reactive.
|
Metal |
Zinc No reaction No reaction Displacement |
Magnesium Displacement No reaction Displacement |
Copper No reaction No reaction No reaction |
Concept insight: The key to this answer is the displacement reaction. A displacement reaction takes place when a more reactive metal is able to displace a less reactive metal from its salt solution.
Solution 2
Concept insight: More reactive a metal is, more likely it is to be corroded. Less reactive a metal is, less likely it is to get corroded.
Solution 3
Concept insight: For answering this question, write the comnplete definition of alloys with all the key words - homogeneous mixture, two or more elements.
Metals and Non-metals Exercise 56
Solution 1
Concept insight: The key to this answer is displacement reaction. A displacement reaction takes place when a more reactive metal is able to displace a less reactive metal from its salt solution.
Solution 2
Concept insight: We can also apply grease and paint to prevent iron from rusting. However, in case of iron frying pan, grease and paint cannot be applied because when the pan will be heated and washed again and again, the coating of grease and paint would get destroyed.)
Solution 3
Concept insight: The key to this answer is to find which oxide has a high melting point and is also soluble in water.
Solution 4
Concept insight: Food cans are coated with tin and not with zinc because zinc is more reactive than tin.
Solution 5
(b) The above tests are useful in distinguishing between metals and non-metals in some cases but not all because there are exceptions also. These tests are based on physical properties and no chemical reactions are involved in these tests.
Concept insight: A hammer can be used to test whether the element is malleable or not. If it can be beaten into thin sheets, it is malelable. Most metals are malleable. Similarly, most metals are good conductors of electricity. So, a battery, bulb, wires, and a switch can be used to set up a circuit.
Solution 6
They react with both acids as well as bases to form salts and water.
Examples: aluminium oxide (Al2O3), zinc oxide (ZnO)
Concept insight: In this answer, you should write the definition of amphoteric oxide. Remember that it shows the properties of both acidic and basic oxides.
Solution 7
Concept insight: For this question, remember that a displacement reation will take place if a more reactive element displaces a less reactive element from its salt solution.
Metals and Non-metals Exercise 57
Solution 8
Anode
Cathode
Electrolyte
Concept insight: Remember CP cathode - pure. So, Anode will be impure.
Solution 9
(ii) Since the gas is sulphur dioxide (SO2), it turns moist blue litmus paper to red because sulphur dioxide reacts with moisture to form sulphurous acid.
(b) S (s) + O2 (g)
Sulphur dioxide
Concept insight: Remember that sulphur when reacted in the presence of air will form its oxide. Also, since sulphur is a non- metal it will turn wet blue litmus paper red.
Solution 10
(i) Oiling, greasing, or painting: By applying oil, grease, or paint, the surface becomes water proof and the moisture and oxygen present in the air cannot come into direct contact with iron. Hence, rusting is prevented.
(ii) Galvanisation: An iron article is coated with a layer of zinc metal, which prevents the iron to come in contact with oxygen and moisture. Hence, rusting is prevented.
Concept insight: For answering this question, mention any two ways for prevention of rusting.
Solution 11
Non-metals combine with oxygen to form acidic or neutral oxides.
2 C (s) + ½ O2 (g)
Neutral oxide
S (s) + O2 (g)
Acidic oxide
Concept insight: Remember that non-metals combine with oxygen to form acidic or neutral oxides.
Solution 12
(b) Sodium, potassium, and lithium are very reactive metals and react very vigorously with air as well as water. Therefore, they are kept immersed in kerosene oil in order to prevent their contact with air and moisture.
(c) Though aluminium is a highly reactive metal, it is resistant to corrosion. This is because aluminium reacts with oxygen present in air to form a thin layer of aluminium oxide. This oxide layer is very stable and prevents further reaction of aluminium with oxygen. Also, it is light in weight and a good conductor of heat. Hence, it is used to make cooking utensils.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction because metals can be easily extracted from their oxides rather than from their carbonates and sulphides
Concept insight: For answering reasoning questions, always write the exact answer of what is required. First read the question carefully, think about the answer and then explain.
Solution 13
Concept insight: For answering this question, recall that tarnishing is corrosion, how is corrosion caused and how can lemon or tamarind juice remove corrosion.
Solution 14
| Metal | non-metal |
| Metals are electropositive. | Non-metals are electronegative. |
|
They react with oxygen to form basic oxides.
4 Na + O 2
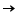 2 Na 2 O 2 Na 2 O |
They react with oxygen to form acidic or neutral oxides.
C + O2
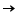 CO2 CO2 |
|
They react with water to form oxides and hydroxides. Some metals react with cold water, some with hot water, and some with steam.
4 Na + 2 H2O
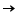 2 NaOH + H2 2 NaOH + H2 |
They do not react with water. |
|
They react with dilute acids to form a salt and evolve hydrogen gas. However, Cu, Ag, Au, Pt, Hg do not react.
2 Na + 2 HCl
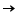 2 NaCl + H2 2 NaCl + H2 |
They do not react with dilute acids. These are not capable of replacing hydrogen. |
| They react with the salt solution of metals. Depending on their reactivity, displacement reaction can occur. | These react with the salt solution of non-metals. |
CuSO4 + Zn 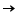 ZnSO4 + Cu ZnSO4 + Cu |
|
They act as reducing agents (as they can easily lose electrons). Na 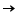 Na+ + e- Na+ + e- |
These act as oxidising agents (as they can gain electrons). ClSub>2 + 2e- 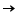 2 Cl- 2 Cl- |
Concept insight: For this question, write all the chemical properties of metals and non- metals.
Solution 15
Concept insight: For answering this question, recall that what is it which reacts with gold.
Solution 16
a. Copper is a better conductor of heat as compared to steel.
b. Copper resists corrosion but steel is not resistant to corrosion.
Concept insight: For answering this question, think that what properties of a substance will be required for a substance to be used in a hot water tank and whether these properties are in copper.


