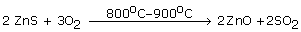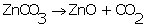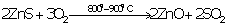Class 10 SELINA Solutions Chemistry Chapter 7 - Metallurgy
Metallurgy Exercise Ex. 7A
Solution 1
(a) Three classes in which elements are classified are:
Metals , Non-metals and Metalloids Copper was the first metal used by man.
(b) The metal which is present in abundance in earths crust is aluminium.
The non-metal which is present in abundance in the earth crust is oxygen.
Solution 2
(a) The metal which is a constituent of blood pigment is Iron (Fe)
(b) The metal which is a constituent of plant pigment is Magnesium (Mg).
Solution 3
(a) Nitrogen: It is used to preserve food.
(b) Hydrogen: It is used in the hydrogenation of vegetable oils to make ghee.
(c) Carbon: It is essential for the growth and development of living beings.
Solution 4
(a) Alkali metals: They are placed in IA group, the first column on the left of the periodic table.
(b) Alkaline earth metal: They are placed in IIA group, the second column on the left of the periodic table.
(c) VIIA
(d) Aluminium: It is placed in IIIA group present on the right of periodic table.
Solution 5
(a) Bromine
(b) Metalloids: Boron (B), Silicon (Si)
(c) Aluminium
(d) Potassium , sodium
(e) Hydrogen
(f) Carbon
Solution 6
Acidic oxide(D)
Discharged at anode (F)
Covalent chlorides (I)
5,6,7 valence electrons (L)
Brittle(C)
Solution 7
(a) Alkali metals like sodium and potassium are kept in kerosene as they react with moisture and air.
(b) Hydrogen, a non-metal, has been included in the metal activity series because it can form a positive ion. It would occupy the position based on its formation of the positive ion.
(c) Gold occupies the lowest position in the metal activity series. This means it will not react with other molecules easily.
Gold is a noble element and will not form new compounds easily. Hence gold ornaments look new even after several years of use.
Solution 8
a. does not react with dilute hydrochloric acid:
Copper
b. can form 2+ and 3+ ions:
Iron (Fe)
c. Metals in the decreasing order of reactivity:
Na > Mg > Zn > Fe > Cu
Solution 9
(a) A metal which occurs as sulphide is lead.
(b) A metal which occurs as halide is silver.
(c) A metal which occurs as carbonate is zinc.
(d) A metal which occurs as oxide is iron.
Solution 10
(a) Minerals are naturally occurring compounds of metals which are generally present with other matter such as soil, sand, limestone and rocks. Ores are those minerals from which the metals are extracted commercially at low cost and comfortably. All ores are minerals, but all minerals are not necessarily ores.
(b) Ores are those minerals from which the metals are extracted commercially at low cost and with minimum effort. A metallic compound is a compound that contains one or more metal elements. Examples: AgNO3 - Silver nitrate is a metallic compound.
Solution 11
The metals that can be extracted from the following ores are:
(a) Bauxite- Aluminium
(b) Calamine- Zinc
(c) Haematite- Iron
Solution 12
(a) Ore:
Ores are those minerals from which metals are extracted commercially at a comparatively lower coast and with minimum effort.
(b) Gangue
The earthly impurities including silica, mud etc. associated with the ore are called gangue.
Metallurgy Exercise Ex. 7B
Solution 1
(a) Hydraulic method: The difference in the densities of the ore and the gangue is the main criterion.
(b) Forth floatation: This process depends on the preferential wettability of the ore with oil and the gangue particles by water.
(c) Electromagnetic separation: Magnetic properties of the ores.
Solution 2
(a) Roasting and calcinations
(b)
(i) It removes moisture from the ore.
(ii) It expels oxide.
(iii) It oxidises sulphide ores to oxide ores.
Solution 3
(a) The processes involved in
(i) Processes involved in dressing of the ore (concentration) are:
1. Hydrolytic method
2. Magnetic Separation
3. Froth floatation
4. Leaching
(ii) Processes involved in Refining of ores are:
1. Distillation
2. Liquation
3. Oxidation
4. Electro- refining
(b) Potassium and sodium oxides cannot be reduced by carbon, carbon monoxide and hydrogen.
Solution 4
Iron and zinc are quite reactive and hence they do not occur in the free state. The compounds of metals found in nature are their oxides, carbonate and sulphides.
Solution 5
Black copper oxide is reduced to brown/red.
CuO + H2 ![]() Cu + H2O
Cu + H2O
Solution 6
Comparison of roasting and calcinations:
|
Roasting |
Calcination |
|
(i) The ore is heated in the excess of air. (ii) Generally, sulphide ores are roasted, so SO2 is given off.
(iii) Volatile impurities are removed as oxides and the ore becomes porous and more reactive. |
(i) The ore is heated in the absence of air. (ii) Carbonate and hydrated ores are calcined and so,CO2 and water vapours are given off. (iii) Moisture and organic impurities are removed and the ore becomes porous and more reactive. |
Solution 7
(a)Ore of zinc is zinc blende (ZnS).
(b)It is concentrated by Froth floatation process.
(c) Concentrated ore is changed into oxide by heating ZnS in excess of air.
2ZnS + 3O2 ![]() 2ZnO + 2SO2
2ZnO + 2SO2
Solution 8
Oxides of highly active metals like potassium, sodium, calcium, magnesium and aluminium have great affinity towards oxygen and so cannot be reduced by carbon or carbon monoxide or hydrogen.
Metals in the middle of activity series (iron, zinc, lead, copper) are moderately reactive and are not found in oxide form. These are found in nature as sulphides or carbonate. These are first converted into oxides and can be reduced by C, CO or H2.
ZnO + C ![]() Zn + CO
Zn + CO
PbO + CO ![]() Pb + CO2
Pb + CO2
Metals low in the activity series is very less reactive and oxides of these metals are reduced to metals by heating alone.
b. CuO + H2 Cu + H2O
Solution 9
(a)Iron(II) oxide:
4FeO + O2 ![]() 2Fe2O3
2Fe2O3
Fe2O3 + 3CO ![]() 2Fe +3CO2
2Fe +3CO2
(b)Zinc oxide is reduced by coke.
Zn O + C ![]() Zn + CO
Zn + CO
Solution 10
Aluminium has a great affinity towards oxygen and so cannot be reduced by carbon or carbon monoxide. So it is extracted from its oxide by electrolysis.
Metals like copper, lead and iron are placed in the middle of the activity series and re moderately reactive and their oxides can be reduced by carbon, CO and hydrogen.
Mercury and silver are less reactive and are placed lower in the reactivity series. The oxides of these metals are reduced to metals by heating their oxides.
Solution 11
The process used for the concentration of the ore is froth floatation process.
Solution 12 (a)
Roasting is the process of heating concentrated ore to a high temperature in the presence of air.
The ore zinc blende is roasted in order to get zinc oxide.
Example: Zinc sulphides are oxidised to zinc oxide.
![]()
Solution 12 (b)
Calcination is the process of heating the concentrated ore such as carbonate or hydrated oxide to a high temperature in the absence of air.
Example: Metal carbonates get decomposed to produce metal oxides.
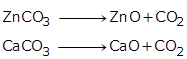
Solution 13
Sodium, potassium and calcium metals are obtained by electrolytic reduction of fused metallic salts.
Solution 14
(a) Reduction of copper oxide:
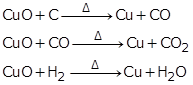
(b) Reduction of iron (III) oxide:
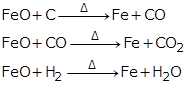
(c) Reduction of lead (II) oxide:
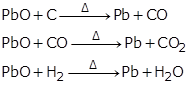
(d) Reduction of zinc oxide:
![]()
Solution 15
(a) The purification depends upon:
1. Nature of metal.
2. Nature of impurities present in the metal.
3. Purpose for which metal is to be used
(b) Methods used for purification are:
1. Distillation
2. Liquation
3. Oxidation
4. Electro-refining
(c)
Solution 16
(a) Option B
The metal other than aluminium which has a strong affinity for oxygen is magnesium.
(b) Option A
A metallic oxide which cannot be reduced by normal reducing agents is zinc oxide.
Solution 17
(a) Usually carbonate ores are subjected to calcination which is done in the absence of air.
(b) Zinc blende is converted to oxide by roasting process.
(c) Froth flotation process is generally used to concentrate sulphide ores.
Metallurgy Exercise Ex. 7C
Solution 1
Position in the Periodic Table: Period 3,Group IIIA(13)
Solution 2
(a)
(i) Ores of aluminium
|
Name |
Chemical name |
Formula |
|
Bauxite |
Hydrated aluminium oxide |
Al3O32H2O |
|
Cryolite |
Sodium aluminium oxide |
Na3AlF6 |
(ii)Ores of iron
|
Name |
Chemical name |
Formula |
|
Red haematite |
Anhydrous ferric oxide |
Fe2O3 |
|
Brown haematite |
Hydrated ferric oxide |
2Fe2O3.3H2O |
(b) Bauxite ore contains approximately 60% aluminium oxide. The rest being sand, ferric oxide and titanium oxide.
(c) Red mud consists of ferric oxide, sand etc. left after bauxite dissolves in NaOH forming sodium aluminate and is removed by filtration.
(i)Ores of aluminium
Solution 3
a.
i. Aluminium oxide dissolves in sodium hydroxide and forms sodium meta aluminate leaving behind insoluble impurities consisting of ferric oxide which is removed by filtration.
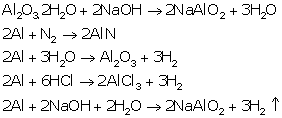
b.
i. The process used for the purification of bauxite is Baeyer's process.
ii. Action of heat on aluminium hydroxide:
![]()
c.
i. Formula of cryolite is
![]()
ii. By dissolving aluminium oxide in cryolite, a conducting solution is produced.
iii. Thick graphite rods are used as the anode. The anode has to be replaced from time to time, as it gets oxidised by evolved oxygen.
iv. Reaction at the cathode:
![]()
v. The cathode is made of carbon.
Solution 4
a. Balanced equations for the purification of bauxite:
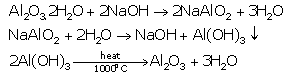
b. Chemicals used for dissolving aluminium oxide: Fluorspar and cryolite Alumina 20%, cryolite 60%, fluorspar 20%
c. At anode: Al 3e− → Al3+
Solution 5
a.
i.
1. Zinc
Zinc blende, Froth flotation, Coke
2. Aluminium
Cryolite, Bauxite, Sodium hydroxide solution
b.
ii.
1. The ore from which aluminium is extracted must first be treated with sodium hydroxide solution so that pure aluminium oxide can be obtained.
2. Pure aluminium oxide is dissolved in cryolite to make a conducting solution.
iii. Formula of cryolite is Na3AlF6.
Solution 6
(a) In the electrolytic reduction of alumina, the graphite (anode) is oxidized by oxygen to CO and further forms CO2, so it is consumed and has to be replaced from time to time.
2C + O2 ![]() 2CO
2CO
2CO + O2 ![]() 2CO2
2CO2
(b) Roasting provides oxygen to convert metallic sulphides into metallic oxide and SO2 which takes place when heated in excess of air.
Carbonate is converted into oxide by loss of CO2 which takes place in the absence of air and when heated strongly.
(c) Aluminium has a great affinity towards oxygen and so cannot be reduced by carbon or carbon monoxide or hydrogen whereas lead oxide can be easily reduced to metal lead by carbon.
PbO + C ![]() Pb + CO
Pb + CO
(d) Aluminium oxide is a very stable compound because of its great affinity for oxygen. It is not reduced easily by common reducing agents such as carbon or hydrogen. Hence, electrolytic reduction is done to obtain aluminium.
(e) Aluminium comes before iron in the metal activity series so it can displace iron from iron salts; thus, food containing iron salts should not be cooked in aluminium utensils.
(f) An anode is made of carbon which gets oxidised in the presence of oxygen to form carbon monoxide which is a neutral gas.
2C + O2 → 2CO
An anode is made of carbon which gets oxidised in the presence of oxygen to form carbon monoxide which is a neutral gas.
2C + O2 → 2CO
(g) Powdered coke is sprinkled on top of the electrolyte. It reduces heat loss by radiation. It also prevents the burning of the anode.
Solution 7
a. Bauxite Aluminium is extracted from bauxite ore. It contains 60% Al2O3
b. Sodium hydroxide The ore from which aluminium is extracted must first be treated with sodium hydroxide solution so that pure aluminium oxide can be obtained.
c. Cryolite It lowers the fusion temperature and enhances conductivity.
d. Graphite Thick graphite rods are used as the anode in electrolytic reduction.
Solution 8
Electrolytic Reduction
(i)It is removal of oxide or halide from a metal.
(ii)Oxides of highly active metals like Na,K,Ca,Mg,Al are reduced by electrolytic reduction of their fused salts.
(iii)Oxides of these metals have great affinity for oxygen than carbon and cannot be reduced by carbon or CO or hydrogen.
Electrolytic refining of metals is the separation of residual impurities like Si and phosphorus.
(i)Presence of other metals and non-metals like Si and phosphorus.
(ii)Unreduced oxides and sulphides of metals.
It depends upon:
(i)Nature of metal
(ii)Purpose for which metal is to be obtained.
(iii)Nature of impurities present.
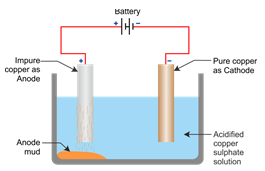
Impure metal is made anode while a thin sheet of pure metal is made cathode and electrolyte used is a salt of solution of a metal to be refined.
Solution 9
The three ways in which metal zinc differs from the non-metal carbon is:
1. Zinc has a valency 2 and carbon has valency 4.
2. Zinc does not form hydride but carbon does (CH4).
3. Oxides of zinc are amphoteric (ZnO) whereas oxides of carbon are acidic (CO2) and neutral (CO).
Solution 10
a. When aluminium is exposed to the atmospheric air, it combines with oxygen and a film of aluminium oxide (Al2O3) is formed at the surface. This hard, tightly adhering film of aluminium oxide prevents corrosion.
b. Aluminium vessels should not be cleaned with powders containing alkalis because aluminium reacts with alkalis to produce meta aluminate.
Solution 11
(a) During the concentration of bauxite ore, aluminium goes in the soluble part because of its amphoteric nature.
(b) In Hoope's process, pure aluminium is collected at the top of the electrolytic cell.
Metallurgy Exercise Ex. 7D
Solution 1
(a) Zinc is electropositive metal than iron, gets oxidized and saves iron. Also zinc forms protective layer of ZnO on iron. This layer is sticky and impervious in nature and protects the iron metal underneath from rusting.
(b) In construction work, the alloy of aluminium–duralumin is used rather than pure aluminium because of the following reasons:
Duralumin is lighter and strong, but aluminium is light and not strong.
Duralumin is unaffected by moist air, while aluminium gets affected by moist air.
Duralumin is corrosion-resistant, while aluminium can undergo corrosion.
Solution 2
Alloy is a homogeneous mixture of two or more metals or of one or more metals with certain non-metallic elements.
The properties of alloys are often greatly different from those of the components.
For example: Gold is too soft to be used without small percentage of copper.
A low percentage of molybdenum improves the toughness and wear resistance of steel.
Bell metal is more sonorous than copper or tin.
Alnico an alloy of aluminium, nickel and cobalt can lift 60 times its own mass.
These added elements improve hardness, wear resistance, toughness and other properties.
Solution 3
The other element in Brass is Zinc.
The other elements in Bronze areTin and Zinc.
Solution 4
(a) Duralumin
(b) Solder
(c) Brass
(d) Brass
Solution 5
A mixture or an alloy of mercury with a number of metals or an alloy such as sodium, zinc, gold and silver as well as with some non-metals is known as amalgam.
Dental amalgam is a mixture of mercury and a silver tin alloy.
Solution 6
(a) Two properties of brass that make it more useful than its components are:
(i) It is malleable and ductile.
(ii) It resists corrosion.
(iii) Can be easily cast.
(b) A metal which forms a liquid alloy at ordinary temperature is sodium.
Solution 7
The constituents of
(a) Duralumin are aluminium (95%), copper (4%), magnesium (0.5%) and manganese (0.5%).
(b) Solder are lead (50%) and tin (50%).
(c) Bronze are copper (80%), tin (18%) and zinc (2%).
(d) Constituents of brass: 60–70% Cu and 40–30% Zn
Solution 8
(a) Mercury
(b) Mercury metal is always present in the amalgam.
(c) Roasting
(d) Slag
(e) Cryolite
(f) Graphite
Solution 9
a. (ii) Copper and zinc
Brass is an alloy of copper and zinc.
b. (iii) Carbon
Steel is an alloy of iron and carbon.
c. (iii) Aluminium brings lightness.
The reason for using aluminium in the alloy duralumin is aluminium brings lightness.
Solution 10
(a) Making electric circuits: Nichrome
(b) Making medals: Bronze
(c) Making parts of watches: Brass
(d) Surgical instruments: Stainless steel
(e) Aircraft: Duralumin and magnalium
Metallurgy Exercise Misc. Ex.
Solution 1
(a) Sodium hydroxide
(b) 2Al(OH)3 ![]() Al2O3 +3H2O
Al2O3 +3H2O
(c) Graphite
(d) Reaction at cathode:
Al3+ + 3e- ![]() Al
Al
(e) Reaction at anode:
Al - 3e- ![]() Al3+
Al3+
Solution 2
(i) A is made of carbon and B is thick graphite rod.
A ![]() Cathode
Cathode
B ![]() Anode
Anode
(ii) Aluminium is formed at electrode A.
(iii) The two aluminium compound in the electrolyte C is Na3AlF6, Al2O3.
(iv) It is necessary to continuously replace electrode B from time to time as it gets oxidized by the oxygen evolved.
Solution 3
|
Use of metal |
Property |
|
Zinc in galvanisation |
Zinc oxidises more readily than iron, thus preventing the rusting of iron. |
|
Aluminium in thermite welding |
Aluminium is a good reducing agent. |
Solution 4
(a) A metal which is found abundantly in the Earth's crust is Aluminium
(b) Differences between calcination and roasting:
|
Calcination |
Roasting |
|
1. The ore is heated in the absence of air. |
1. The ore is heated in excess of air. |
|
2. Moisture and organic impurities are removed and the ore becomes porous and more reactive. |
2. Volatile impurities are removed as oxides (SO2, P2O5, As2O3) and the ore becomes porous and more reactive. |
|
3. Carbonate and hydrated ores are calcined and CO2 or water vapour is given off.
|
3. Sulphide ores are roasted, so SO2 is given off.
|
(c) Froth flotation process is used for the enrichment of sulphide ore.
(d) Ores of iron
|
Name |
Chemical name |
Formula |
|
Red Haematite |
Anhydrous ferric oxide |
Fe2O3 |
|
Brown Haematite |
Hydrated ferric oxide |
2Fe2O3.3H2O |
Ores of aluminum
|
Name |
Chemical name |
Formula |
|
Bauxite |
Hydrated aluminium oxide |
Al3O32H2O |
|
Cryolite |
Sodium aluminium oxide |
Na3AlF6 |
(e) Constituents of the electrolyte for the extraction of aluminium are pure alumina (![]() , cryolite (Na3AlF6) and fluorspar (
, cryolite (Na3AlF6) and fluorspar (![]() ).
).
Solution 2012
a.
i. A metal present in cryolite other than sodium: Aluminium
ii. A metal which is unaffected by dilute or concentrated acids:
Aluminium
iii. A metal present in Period 3, Group 1 of the periodic table:
Sodium (Na)
b.
i. Caustic alkali is added to bauxite ore during extraction as the insoluble part of bauxite is removed and the alumina component is then precipitated.
ii. The reaction is as follows:
Al2O3.2H2O + 2NaOH →2NaAlO2 + 3H2O
iii. Fluorspar is added along with cryolite and alumina because this helps the mixture to fuse at ![]() instead of
instead of![]() , and the aluminium obtained at this temperature is in the liquid state.
, and the aluminium obtained at this temperature is in the liquid state.
Solution 2013
i. Y is the metallic element.
ii. Metal atoms tend to have a maximum of 3 electrons in the outermost energy level.
iii. Non-metallic elements tend to form acidic oxides, while metals tend to form basic oxides.
iv. Non-metallic elements tend to be poor conductors of heat and electricity.
v. Metals tend to lose electrons and act as reducing agents in their reactions with elements and compounds.
Solution 2014
a. The main ore used for the extraction of iron is
Haematite
b. Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
Calcination
c.
i. Brass Main components of brass are copper and zinc.
ii. Duralumin
Main components of duralumin are aluminium, magnesium, copper and manganese.
iii. Bronze
Main components of bronze are copper, zinc and tin.
d.
i. Malleability is the property possessed by metals by which they can be beaten into sheets.
ii. Cryolite is added to lower the fusion temperature of an electrolytic bath in the extraction of aluminium.
iii. Zinc blende (sphalerite) is the ore of zinc containing its sulphide.
Solution 2015
(a)
(i) Na2O
(ii) SO2
(iii)Al2O3
(iv)SiO2
(b) In the extraction of aluminium, the given compounds play the following roles:
(i) Cryolite: It lowers the fusion temperature from 2050°C to 950°C and enhances conductivity.
(ii) Sodium hydroxide:
Two roles are played by sodium hydroxide in the extraction of aluminium.
First, finely grinded bauxite (ore of aluminium) is heated under pressure with conc. caustic soda solution (NaOH solution) for 2-8 hours at 140°C to 150°C to produce sodium aluminate. The chemical equation is as follows:
Al2O3.2H2O + 2NaOH → 2NaAlO2 + 3H2O
Second, on diluting sodium aluminate with water and cooling to 50°C, sodium aluminate is hydrolysed to give aluminium hydroxide as precipitate. Here, the impurities dissolve in sodium hydroxide.
(iii) Graphite: Thick rods of graphite are suspended in the fused electrolyte. They act as an anode where oxygen gas is discharged.
Solution 2016
(a) Conc. caustic soda
(b) ![]()
(c) Cryolite
(d) At the cathode: Al3+ + 3e-→ Al
(e) The anode has to be replaced from time to time as it gets oxidised by oxygen evolved at the anode.