Class 10 SELINA Solutions Chemistry Chapter 6 - Electrolysis
Electrolysis Exercise Intext 1
Solution 1
(a) Powdered sodium chloride (common salt) does not conduct an electric current, but it does so when dissolved in water or when melted.
(b) Molten lead bromide conducts electricity .It is called an electrolyte. It is composed of lead ions and bromide ions. The lead ions are positively charged and are called cations. The bromide ions are negatively charged and are called anions.
(c) Substances which conduct electricity in the solid state are generally metals.
(d) The electron releasing tendency of zinc is more than that of copper.
(e) A solution of HCl gas in water conducts electricity because it ionizes , but a solution of HCl gas in toluene does not conduct an electric current because it does not ionize in toluene.
(f) Pure water consists entirely of molecules.
(g) We can expect that pure water will not normally conduct electricity.
(h) Electrolysis is the passage of electricity through a liquid or a solution accompanied by a chemical change.
Solution 2
(a) Electrolysis: It is the process of decomposition of a chemical compound in aqueous solutions or in molten state accompanied by a chemical change using direct electric current.
(b) Non-electrolyte: It is a compound which neither in solution nor in the molten state allows an electric current to pass through it.
(c) Cation and anion: Atoms which carry positive charge are called cations.
Atoms which carry negative charge are called anions.
(d) Weak electrolyte: Electrolytes which allow small amount of electricity to flow through them and are partially dissociated in fused or aqueous solution are called weak electrolyte.
Solution 3
(a) Difference between Modern explanation and Arrhenius explanation for the theory of electrolysis:
Arrhenius considered that water ionizes electrolytes but Modern theory explained that electrolytes are ionic even in solid state and their ions are held by strong electrostatic forces which make them immobile. Water renders these ions mobility by breaking the electrostatic forces.
(b) Difference between electrolytic dissociation and ionization :
|
Ionisation |
Dissociation
|
|
1. Formation of positively or negatively charged ions from molecules which are not initially in the ionic state. |
1. Separation of ions which are already present in an ionic compound. |
|
2. Polar covalent compounds show ionization. e.g. HCl, H2CO3, NH4OH etc. |
1. Electrovalent compounds show dissociation. e.g. Potassium chloride , lead bromide, etc. |
(c) A cation and anion:
|
Cation |
Anion |
|
1. Are positively charged ions. |
Are negatively charged ions. |
|
2. Migrate to cathode during electrolysis. |
Migrate to anode during electrolysis. |
|
3. Gain electron from the cathode and get reduced to become a neutral atom. |
Lose electrons to the anode and get oxidized to become a neutral atom. |
(d) Electrolytic dissociation and thermal dissociation:
Electrolytic dissociation is the dissociation of an electrovalent compound into ions in the fused state or in aqueous solution state.
Thermal dissociation: Reversible breakdown of a chemical compound into simpler substances by heating it. The splitting of ammonium chloride into ammonia and hydrogen chloride is an example. On cooling, they recombine to form the salt.
(e)
|
Strong Electrolytes |
Weak Electrolytes |
|
Electrolytes which allow a large amount of electricity to flow through them. |
Electrolytes which allow small amounts of electricity to flow through them. |
|
These are good conductors of electricity. |
These are poor conductors of electricity. |
|
These almost completely dissociate in the fused or aqueous solution state. |
These are partially dissociated in the fused or aqueous solution state. |
|
These solutions contain only free mobile ions. |
These solutions contain ions as well as molecules. |
Solution 4
(a) Sodium carbonate
(b) NH4OH
(c) An inert electrode: graphite and Active electrode: silver
(d) H+
(e) Electrode is cathode
(f) Graphite
Solution 5
Electrolysis is a redox process. The reaction at the cathode involves reduction of cations as they gain of electrons while the reaction at anode involves oxidation of anions as they loss of electrons to become neutral.
Example: Dissociation of sodium chloride during electrolysis.
NaCl ![]() Na+
+ Cl-
Na+
+ Cl-
Cathode : Na+ +
e- ![]() Na(reduction)
Na(reduction)
Cl- - e- ![]() Cl(oxidation)
Cl(oxidation)
Cl + Cl ![]() Cl2
Cl2
Overall reaction:2NaCl ![]() 2Na + Cl2
2Na + Cl2
Solution 6
a. dilute hydrochloric acid, dilute sulphuric acid, sodium acetate
b. acetic acid, ammonium hydroxide
c. carbon tetrachloride
Solution 7
a. Copper metal is a solid and has no mobile ions, whereas an electrolyte should dissociate into oppositely charged ions to conduct the electric current.
b. In solid sodium chloride, Na+ and Cl- ions are not mobile to conduct the electric current.
Solution 8
i. Molten ionic compound - strong electrolyte
ii. Carbon tetrachloride - non-electrolyte
iii. Aluminium wire - metallic conductor
iv. A solution containing solvent molecules, solute molecules and ions formed by the dissociation of solute molecules. - weak electrolyte
v. A sugar solution with sugar molecules and water molecules. - non-electrolyte
Solution 9
D. Sodium hydroxide
An electrolyte which completely dissociates into ions is Sodium hydroxide.
Electrolysis Exercise Intext 2
Solution 1
(a) Glucose, Kerosene
(b) NaCl and NaOH
(c) CH3COOH and NH4OH
Solution 2
(a) OH-
(b)Ag+
Solution 3
(a) Zn occurs readily as ion whereas Cu occurs more readily as metal in nature.
(b) Copper is above silver in the electrochemical series and is thus more reactive than silver. So, copper displaces silver from silver nitrate. Hence, we cannot store AgNO3 solution in copper vessel.
Cu +AgNO3 ![]() Cu(NO3)2 + 2Ag
Cu(NO3)2 + 2Ag
(c) Copper is more active than Ag.
Solution 4
(a) By treating its salt with a more reactive metal.
(b) By supplying two electrons to Cu+2
Cu+2 + 2e-![]() Cu
Cu
Solution 5
In the aqueous state, the slightly negatively charged oxygen atoms of the polar water molecule exerts a pull on the positively charged sodium ions. A similar pull is exerted by the slightly charged hydrogen atoms of the water on the negatively charged chloride ions. Thus the ions become free in solution. These free ions conduct electricity.
In the molten state, the high temperatures required to melt the solid weakens the bond between the particles and the ions are set free.
Solution 6
(a) Two anions are ![]() and OH-.
and OH-.
(b) OH- is discharged at anode and the main product of the discharge of OH- is O2
Reaction is :
OH-![]() OH +e-
OH +e-
4OH ![]() 2H2O + O2
2H2O + O2
(c) The product formed at cathode is hydrogen. The reaction is :
H+ + e- ![]() H
H
H + H ![]() H2
H2
(d) No change in colour is observed.
(e) Dilute sulphuric acid catalyse the dissociation of water molecules into ions, hence electrolysis of acidified water is considered as an example of catalysis.
Solution 7
a. A = Platinum anode, B = Platinum or copper cathode
b. A = Platinum anode
Solution 8
The addition of sulphuric acid causes dissociation of water into H+ ions and OH- ions.
Solution 9(a)
(a) Electrolyte
(b) Nickel
(c) Cathode
(d) Anode
(e) Cations
Solution 9(b)
cathode, anode
Solution 9(c)
Electrolysis of acidulated water is an example of redox reaction.
Solution 10
(a) Cane sugar is a compound which does not have ions even in solution and contains only molecules. Hence, it does not conduct electricity. On the other hand, sodium chloride solution contains free mobile ions and allows electric current to pass through it. This makes it a good conductor of electricity.
(b) Hydrochloric acid is a strong electrolyte and dissociates completely in aqueous solution. The solution contains free mobile ions which allow electric current to pass through it. Hence, hydrochloric acid is a good conductor of electricity.
(c) Hydrogen is placed lower in the electrochemical series and sodium is placed at a higher position. This is because H+ ions are discharged more easily at the cathode than Na+ during electrolysis and gains electrons more easily.
Therefore, H+ ion is reduced at the cathode and not Na+ ion.
(d)Copper is placed below hydrogen in the activity series. Cu2+ on reduction is discharged as metallic copper in preference to hydrogen.
(e) Since hydrogen is much below sodium in the activity series, hydrogen is discharged at the cathode in preference to sodium.
(f)Zinc is more reactive than hydrogen, so it displaces hydrogen from acids, but copper is less reactive than hydrogen, so it does not liberate hydrogen from acids.
Electrolysis Exercise Ex. 6
Solution 1
(a) During electrolysis of lead bromide, there is loss of electrons at anode by bromine and gain of electrons at cathode by lead. Thus oxidation and reduction go side by side. Therefore, it is a redox reaction.
![]()
(b) The blue colour of copper ions fades due to decrease in Cu+2 ions and finally the solution becomes colourless as soon as Cu+2 ions are finished.
(c) Lead bromide dissociate into ions in the molten state whereas it does not dissociate in solid state. The ions become free when lead bromide is in molten state but in the solid state the ions are not free since they are packed tightly together due to electrostatic force between them. Therefore, lead bromide undergoes electrolytic dissociation in the molten state.
(d) Aluminium has great affinity towards oxygen, so it is not reduced by reducing agent. Therefore it is extracted from its oxide by electrolytic reduction.
(e) As per electrolytic reactions, 4H+1 are needed at cathode and 4OH- at the anode and two molecules of water are produced at the anode. Hence for every two molecules of water, two molecules of hydrogen and one molecule of oxygen are liberated at the cathode and anode respectively.
![]()
(f) This is because HNO3 is volatile.
(g) Ammonia is a covalent compound. Therefore, it is unionized in the gaseous state but in the aqueous solution it gives NH4OH which is a weak electrolyte and dissociates into ions.
(h) Graphite is unaffected by the bromine vapours.
(i) Silver nitrate is not used as electrolyte for electroplating with silver because the deposition of silver will be very fast and hence not very smooth and uniform.
(j) Carbon tetrachloride is a liquid and does not conduct electricity because it is a covalent compound and there are no free ions present and contain only molecules.
(k) Potassium is not extracted from its aqueous salt solution by electrolysis as it can react with water.
Solution 2 (a)
|
|
Anode |
Electrolyte |
Cathode |
|
(i) Silver plating of a spoon |
Plate of pure clean silver |
Solution of potassium argentocyanide |
Article to be electroplated |
|
(ii) Purification of copper |
Impure copper |
Solution of copper sulphate and dilute sulphuric acid |
Thin strip of pure copper |
|
(iii)Extraction of sodium |
Graphite |
Fused sodium chloride |
Iron |
Solution 2 (b)
|
|
Reactions taking place at anode |
|
Silver plating of a spoon |
Ag - e- - → Ag+ |
|
Purification of copper |
Cu - 2e- - → Cu2+ |
|
Extraction of sodium |
Cl- - e-- → Cl Cl + Cl → Cl2 |
Solution 3
(a)
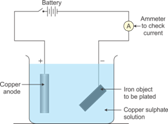
(b) CuSO4 is preferred as an electrolyte.
(c) The copper anode continuously dissolves as ions in solution and is replaced periodically. The electrolyte dissociates into Cu+2 ions which migrate towards the iron object taken as the cathode and are deposited as neutral copper atoms on the cathode.
Electrolyte: Aqueous solution of nickel sulphate
Dissociation: CuSO4 ![]() Cu2+ + SO42-
Cu2+ + SO42-
H2O ![]() H+ + OH-
H+ + OH-
Electrodes:
Cathode: Article to be electroplated
Anode: Block of pure copper
Electrode reactions:
Reaction at cathode: Cu2+ + 2e-→ Cu (deposited)
Reaction at anode: Cu - 2e-→ Cu2+
Solution 4
(a) X![]() X2+ +2e-,Y + 3e-
X2+ +2e-,Y + 3e-![]() Y3-
Y3-
(b) Y2 + 3X![]() X3Y2
X3Y2
(c) Cathode, Anode
Solution 5
i. Electroplating of metals
ii. Electrorefining of metals
Solution 6
(a) Non-electrolyte contains molecules.
(b) Molecules of HX and H+ and X- ions.
(c) Loss
(d) The electrolyte used for the purpose must contain the ions of metal which is to be electroplated on the article.
Solution 7
AgNO3 solution
Solution (2008)
(a) The reaction takes place at anode. This is an example of oxidation.
(b) Cu+2 will discharge easily at cathode.
Reaction at cathode:
Cu+2 +2e- ![]() Cu
Cu
(c) Carbon tetrachloride is a non-electrolyte because it is a covalent compound. It does not ionize and hence do not conduct electricity.
(d) Correct option: D - Lead is deposited at cathode during the electrolysis of molten lead bromide.
Solution 2009(a)
D. CH3COOH
Solution 2009(b)
Molten lead bromide conducts electricity.
Solution 2009(c)
i. Complex salt
ii. On using silver nitrate, the deposition of silver on the cathode is very fast and hence not very smooth and uniform because it is a strong electrolyte.
iii. A long current for a longer time should be used.
iv. Ag+ + e- → Ag
v. Ag - e- → Ag+
Solution 2009(d)
i. Ni2+ ions
ii. Oxygen gas, when an inert electrode is used.
Solution 2010(a)
iv. Lead [II] bromide
Solution 2010(b)
i.
A. Aqueous solution of nickel sulphate with few drops of dil. sulphuric acid
B. Article (e.g. key chain)
C. Pure nickel
ii.
A. Ni2+ + 2e- →Ni
B. Ni → Ni2+ + 2e-
Solution 2010(c)
Cell A contains sodium chloride solution which is a strong electrolyte and contains only ions. So, it conducts electricity and the bulb glows brightly.
Cell B contains both ions and molecules. So, there are few ions to conduct electricity and the bulb glows dimly.
Cell C contains sugar solution which is a non-electrolyte and does not contain ions. So, it is a bad conductor of electricity and the bulb does not glow.
Solution 2011(a)
Dilute sulphuric acid catalyses dissociation, so electrolysis of acidified water is considered an example of catalysis.
Solution 2011(b)
i. Red shiny metal deposits at the cathode.
ii. The colour of the electrolytes changes gradually from blue to colourless.
iii. At the cathode:
Cu2+ + 2e- → Cu
Reaction at the anode:
OH- → OH + e-
4OH → 2H2O + O2
Solution 2011(c)
|
Copper sulphate solution |
Copper metal |
|
Conduction of electricity is due to the flow of ions. |
Conduction of electricity is due to the flow of electrons. |
|
It is an aqueous solution of an ionic compound. |
It is a metal in the solid state. |
|
It undergoes a chemical change. |
It remains unchanged chemically. |
Solution 2012(a)
iv. Aq. acetic acid
Solution 2012(b)
Ammonium hydroxide solution - Contains ions and molecules
Dilute hydrochloric acid - Contains only ions
Carbon tetrachloride - Contains only molecules
Solution 2012(c)
An aqueous solution of sodium chloride contains free sodium ions and chloride ions. It thus allows a large amount of electricity to flow through and the bulb glows brightly.
Solution 2012(d)
i. Cu2+
ii. Pt
iii. Cu2+
iv. H+
v. Ag
Solution 2013(a)
Dark reddish brown fumes of bromine evolve at the anode and greyish white metal lead is formed on the cathode.
Solution 2013(b)
i. Liquid carbon tetrachloride
Solution 2013(c)
i. The right electrode is the anode and oxidising electrode. Cu → Cu2+ + 2e- losing electrode.
ii. Reaction at the anode: Cu → Cu2+ + 2e-
Reaction at the cathode: Cu2+ + 2e-→ Cu
iii. The anode dissolves and anode mud containing precious metal is recovered.
Solution 2013(d)
Hydrogen chloride
Solution 2014(a)
iii. A silver grey deposit at cathode and reddish brown fumes at anode.
Solution 2014(b)
iii. Sodium argentocyanide solution
Solution 2014(c)
Galvanisation
Solution 2014(d)
Acidified aqueous copper sulphate solution is electrolysed with copper electrodes by electrolysis. The electrolysis of an aqueous solution of copper sulphate using copper electrodes (i.e. using active electrodes) results in transfer of copper metal from the anode to the cathode during electrolysis. The copper sulphate is ionised in the aqueous solution.
Copper sulphate solution is ionised by the following chemical equation:
CuSO4 → Cu2+ + SO42-
The positively charged copper ions migrate to the cathode, where each gains two electrons to become copper atoms which are deposited on the cathode.
Cu2+ + 2e-→ Cu
Hence, the colour of copper sulphate changes from
blue to colourless.
Solution 2014(e)
Cathode (Reducing electrode): At the cathode, the cations gain electrons to form neutral atoms. As electrons are gained, the ion is said to be reduced. Anode (Oxidising electrode): At the anode, the anions lose electrons to form neutral atoms. As electrons are lost, the ion is said to be oxidised.
Solution 2014(f)
i. Positive sodium ions and negative hydroxide ions
ii. Hydrogen ions and carbonate ions
iii. Glucose, fructose and galactose
Solution 2014(g)
i. At the cathode: M+ + 1e-→ M
ii. At the anode: Oxygen gas
Solution 2015 (a)
Copper anode itself ionises to give Cu2+ ions.
Cu - 2e- → Cu2+
Solution 2015 (b)
During the electrolysis of molten lead bromide, a graphite anode is preferred because graphite remains unaffected by the reactive bromine vapours which are released at the anode.
Solution 2015 (c)
In the electrolysis of molten lead bromide, the following reactions take place:
At the cathode: Pb2+ (l) + 2e- → Pb(l)
At the anode: 2Br- (l) → Br2 (g) + 2e-
Lead (II) ions (Pb2+) are attracted to the negative electrode, and the Pb2+ ions are forced to accept two electrons. Pb2+ ions are reduced. Bromide ions (Br-) are attracted to the positive electrode, and the bromide ions are forced to give away their extra electron to form bromine atoms. Thus, bromide ions are oxidised. So, electrolysis of molten lead bromide is a redox reaction.
Solution (2016)
(a) Electrostatic forces of attraction between ions in the solid state are very strong. These forces weaken in the fused or solution state. Hence, ions become mobile.
(b) If silver nitrate solution is used directly instead of double cyanide of silver and sodium, silver will deposit very fast and its deposition will not be smooth and uniform.
(c) Copper has no mobile electrons in the solid state and an electrolyte should dissociate into oppositely charged ions to conduct electricity.
Hence, copper is a non-electrolyte.

