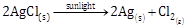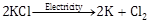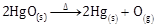Class 9 SELINA Solutions Chemistry Chapter 2 - Chemical Changes and Reactions
Chemical Changes and Reactions Exercise Ex. 2(A)
Solution 1
(a) A chemical reaction is the process of breaking the chemical bonds of the reacting substances (reactants) and making new bonds to form new substances (products).
(b) Conditions necessary for a chemical change or reaction are
(i) Evolution of gas
(ii) Change of colour
(iii) Formation of precipitate
(iv) Change of state
Solution 2
- A chemical change is a permanent change in which the composition of a substance which results in the formation of substance with different chemical composition and properties.
- A chemical bond is the force which holds the atoms of a molecule together as in a compound.
- Formation of gas bubbles in a liquid during a reaction is called effervescence.
- Chemical reactions which are characterised by the formation of insoluble solid substances are called precipitates.
Solution 3
(a) ![]()
(b) ![]()
(c) ![]()
(d) ![]()
(e) ![]()
(f) ![]()
(g) ![]()
Solution 4
(a) It is a reaction which occurs with absorption of light energy.
![]()
(b) It is a reaction which occurs with absorption of electrical energy.
![]()
Solution 5
a.
i. ![]()
ii. ![]()
b.
i. 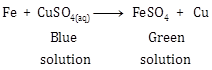
ii. 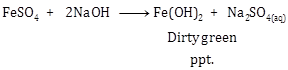
c. ![]()
d. ![]()
e. 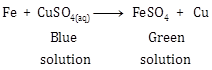
Solution 6
(a) ![]()
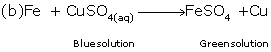
(c) ![]()
(d) ![]()
Solution 7
- Silver nitrate solution is kept in brown bottles in the laboratory because it decomposes in the presence of light.
- Molybdenum increases the efficiency of the catalyst iron used in the manufacture of ammonia.
- This is because the blue colour of the copper sulphate solution fades and eventually turns into light green due to the formation of ferrous sulphate.
- Copper displaces hydrogen from sulphuric acid and forms blue-coloured copper sulphate and hydrogen gas is evolved.
Chemical Changes and Reactions Exercise Ex. 2(B)
Solution 1
a.
(ii) No net energy change is involved.
b.
(iii) Exchange of ions of AB and CD
c.
(iv) Double displacement reaction
d.
(i) Carbon dioxide
Solution 2
|
a. Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) |
i. Displacement reaction |
|
b. |
ii. Photochemical decomposition |
|
c. |
iii. Electrolytic decomposition |
|
d. |
iv. Thermal decomposition |
Solution 3
- Displacement
- Double decomposition
- Accelerates, decelerates, unaffected
- Blue, white
Solution 4
When hydrogen burns in oxygen, water is formed - Combination Reaction
When electricity is passed through water, hydrogen and oxygen are given out - Decomposition Reaction.
Solution 5
(a) Double decomposition:
This is a type of chemical change in which two compounds in a solution react to form two new compounds by mutual exchange of radicals.
![]()
(b) Thermal dissociation
A reversible decomposition reaction brought about only by heat is thermal dissociation.
Example:
Heat some solid ammonium chloride in a test tube. Two colourless gases, ammonia and hydrogen chloride, are produced. As these gases move up to the upper part of the test tube which is cooler, they combine to form ammonium chloride, which appears as a white sublimate on the upper cooler side of the test tube.
NH4Cl ⇌ NH3↑ + HCl↑
(c) Reversible reaction
A chemical reaction in which the direction of a chemical change can be reversed by changing the conditions under which the reaction is taking place is called a reversible reaction.
CuSO4.5H2O(s) ⇋ CuSO4(s) + 5H2O(g)
(d) Displacement
It is a chemical change in which a more active element displaces a less active element from its salt solution.
CuSO4 + Zn → ZnSO4 + Cu
Solution 6
A reaction in which two or more substances combine together to form a single substance is called a synthesis or combination reaction.
A + B → AB
In the above reaction, substances A and B combine to give a molecule of a new substance, AB.
Carbon burns in oxygen to form a gaseous compound, carbon dioxide.
C + O2![]() CO2
CO2
Solution 7
A decomposition reaction brought about by heat is known as thermal decomposition.
2HgO(s) ![]() 2Hg(s) + O2(g)
2Hg(s) + O2(g)
A simultaneous reversible decomposition reaction brought about only by heat is thermal dissociation.
NH4Cl ⇋ NH3 +HCl
Solution 8
- The reaction between an acid and a base to form salt and water only is referred to as a neutralisation reaction.
- NaOH + HCl → NaCl + H2O
- Applications of neutralisation reactions:
- When someone is stung by a bee, formic acid enters the skin and causes pain, which can be relieved by rubbing the spot with slaked lime or baking soda, both of which are bases.
- Acid which is accidentally spilled on to our clothes can be neutralised with ammonia solution.
- If soil is somewhat acidic and thus unfavourable for growing of certain crops, slaked lime is added to neutralise the excess acid.
Solution 9
Precipitation reaction
The insoluble solid formed during the double displacement reactions is called a precipitate. Reactions in which a precipitate is formed as one of the products are also called precipitation reactions.
Sodium sulphate reacts with barium chloride to form barium sulphate and sodium chloride solution.
Na2SO4 (aq) + BaCl2 → BaSO4(s) + 2NaCl (aq)
Sodium Barium Barium Sodium
sulphate chloride sulphate chloride
Solution 10
- Reactions in which two compounds react with each other and exchange their ions to form two new compounds, are called double displacement reaction. In these reactions, two compounds swap components, in the format:
AB + CD → AD + CB
- The reactants of double displacement reaction are in aqueous form i.e. solution and are often accompanied by precipitation. In other words, a solid product separates or settles at the bottom of the solution.
FeS(s) + H2SO4 (aq) ⟶ FeSO4(aq) + H2S↑ is a an example of double displacement reaction, where a gas is evolved.
Solution 11 (a)
Decomposition is the breaking up of a compound either into elements or simpler compounds such that these products do not combine to form the original compound.
Solution 11 (b)
(i) heat
(a) ![]()
(b) ![]()
(ii) electricity
(a) ![]()
(b) ![]()
(iii) Sunlight
![]()
Solution 12
- Cl2 + 2KBr → 2KCl + Br2
Displacement reaction
- NaOH + HCl → NaCl + H2O
Neutralisation reaction
- 2HgO → 2Hg + O2
Decomposition reaction
- Fe + CuSO4 → FeSO4 + Cu
Displacement reaction
- PbO2 + SO2 → PbSO4
Combination reaction
- 2KClO3 → 2KCl + 3O2
Decomposition reaction
- 2H2O2 → 2H2O + O2
Decomposition reaction
- KNO3 + H2SO4→ HNO3 + KHSO4
Double decomposition reaction
- CuO+H2 → Cu+ H2O
Displacement reaction
- CaCO3 → CaO+ CO2
Decomposition reaction
- NH4Cl → NH3 + HCl
Decomposition reaction
- PbO + 2HNO3 → Pb(NO3) + 2H2O
Neutralisation reaction
- AgNO3 + NaCl → AgCl + NaNO3
Double decomposition reaction
Chemical Changes and Reactions Exercise Ex. 2(C)
Solution A. 1
Correct option: (ii)- Iron is magnetized
Solution A. 2
Correct option: (iii)- Decomposition
Solution A. 3
Correct option: (iii)- Direct combination reaction between two elements
Solution A. 4
Correct option: (iv)- Double decomposition
Solution A. 5
Correct option: (iii) A = Cu and B = CuO
Solution A. 6
Correct option: (iii)- Combustion of LPG
Solution A. 7
Correct option: (ii)- Double decomposition reaction
Solution A. 8
Correct option: (ii)- Double decomposition reaction
Solution B. 1
(a) Sodium carbonate
(b) Sodium nitrate
(c) Zinc carbonate
(d) Lead nitrate
Solution B. 2
The given reaction is displacement reaction where iron has replaced copper from its copper sulphate salt. Thus, iron is more reactive than copper.
Solution C. 1
(a) It is a reaction which occurs with absorption of light energy.
Example: Photosynthesis
![]()
(b) It is a reaction which occurs with absorption of electrical energy.
Example:
Acidulated water breaks into hydrogen and oxygen.
![]()
Solution C. 2
(a) C + O2 → CO2 + Heat
(b) C + 2S ![]() CS2
CS2
(c) N2 + 3H2![]() 2NH3
2NH3
Solution C. 3
Iron is more reactive than copper. Hence, iron is able to displace copper from its salt i.e., copper sulphate salt in aqueous solution.
CuSO4 + Fe → FeSO4 + Cu
Copper sulphate solution is blue in colour but when iron filings are added to it, solwly iron replaces copper from its salt to form iron sulphate and soon the blue colour fades. Copper metal is displaced and settles down in the beaker.
Solution C. 4
(a) Single displacement reaction
CuSO4 + Fe → FeSO4 + Cu
(b) Double displacement reaction
BaCl2 (aq) + Na2SO4 (aq) → BaSO4 (s) + 2NaCl (aq)
white
(c) Photochemical reaction
![]()
(d) Thermal decomposition reaction
![]()
(e) Electrolytic decomposition reaction
![]()
(f) Combination reaction between two compounds
![]()
Solution C. 5
A chemical reaction is the process of breaking the chemical bonds of the reacting substances (reactants) and making new bonds to form new substances (products).
A chemical change or chemical reaction occurs when particles collide. Collisions occur when reactants are in close contact or by supply of energy.
Solution C. 6
(a)
(i) Change of state
Ammonia gas reacts with HCl gas to give solid ammonium chloride.
NH3(g) + HCl(g) ⇋ NH4Cl(s)
(ii) Formation of precipitate
When a solution of silver nitrate is added to a solution of sodium chloride, a white insoluble substance, silver chloride, is formed.
AgNO3(aq) + NaCl(aq) → AgCl(aq) + NaNO3(aq)
(b)
Exothermic reaction:
When carbon burns in oxygen to form carbon dioxide, a lot of heat is produced.
C + O2 → CO2 + Heat
Endothermic reaction:
When carbon is heated with sulphur at high temperature, liquid carbon disulphide is formed.
C + 2S ![]() CS2
CS2
(c) Colour change
A few pieces of iron are added into a blue coloured copper sulphate solution; the blue colour of copper sulphate fades and eventually turns into light green due to the formation of ferrous sulphate.
Fe + CuSO4 → FeSO4 + Cu
Solution C. 7
A chemical change is a permanent change in which the chemical composition of a substance is changed and a new substance is formed.
Examples:
Heating of copper carbonate
Formation of curd from milk
Solution C. 8
Exothermic reaction:
A chemical reaction in which heat is given out is called an exothermic reaction.
Example:
When carbon burns in oxygen to form carbon dioxide, a lot of heat is produced.
C + O2 → CO2 + Heat
When hydrogen is burnt in oxygen, water is formed and heat is released.
2H2 + O2 ![]() 2H2O + Heat
2H2O + Heat
Endothermic reaction:
A reaction in which heat is absorbed is called endothermic reaction.
Example:
When carbon is heated with sulphur at high temperature, liquid carbon disulphide is formed.
C + 2S ![]() CS2
CS2
When nitrogen and oxygen are heated together to a temperature of about 3000°C, nitric oxide gas is formed.
N2 + O2![]() 2NO
2NO
Solution C. 9
In every chemical change, change in energy is involved.
There is a difference between the chemical energies of the reactants and products. It involves the breaking up of chemical bonds between the atoms resulting in the absorption of energy in the form of heat and simultaneous formation of bonds with the release of energy.
Solution D. 1
The main characteristics of chemical reactions:
(i) Evolution of gas: In many chemical reactions, one of the products is a gas.
For example, when zinc reacts with dilute sulphuric acid, hydrogen gas is evolved with is a gas.
Zn + H2SO4 → ZnSO4 + H2↑
(ii) Change of colour : Certain chemical reactions are characterized by a change in the colour of the reactants.
For example, when a few pieces of iron are dropped into a blue coloured copper sulphate solution, the blue colour of the solution fades and eventually turns into light green due to the formation of ferrous sulphate.
Fe + CuSO4(aq) → FeSO4 (aq) + Cu
Blue soln. Green Soln.
(iii) Formation of precipitates: Certain chemical reactions ae characterized by the formation of insoluble solid substances called precipitates.
For example, when a solution of silver nitrate is added to a solution of sodium chloride, a white insoluble substance (precipitate), silver chloride, is formed.
AgNO3(aq) + NaCl(aq) → AgCl↓ + NaNO3(aq)
(iv) Change of state: In many chemical reactions, a change of state is observed. A reaction might start with gaseous or liquid reactants and end up with solid products, or vice versa.
For example, Ammonia gas reacts with hydrogen chloride gas to produce solid ammonium chloride.
NH3(g) + HCl(g) ⇌ NH4Cl(s)
Solution D. 2
Endothermic reactions are those reactions which absorb heat from the surrounding for the reaction to take place, consequently decreasing the temperature of the surrounding.
Endothermic reactions draw in heat from their surroundings, causing their surroundings to cool down.
For example:
Formation of carbon disulphide: When carbon is heated with sulphur at high temperature, liquid carbon disulphide is fomred.
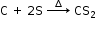
Exothermic reactions are those reactions which liberate heat during the reaction thus raising the temperature of the surrounding.
Exothermic reactions are spontaneous causing their surroundings to warm up as they release heat energy.
For example:
When carbon burns in oxygen to form carbon dioxide, a lot of heat is produced.
C + O2 → CO2 + Heat
Solution D. 3
(a) NaCl(aq) + AgNO3(aq) → AgCl(aq) + NaNO3(aq)
(b) Pb(NO3)2 + 2KI →2KNO3 + PbI2
(c) CuCO3![]() CuO(s) + CO2(g)
CuO(s) + CO2(g)
(d) 2Pb(NO3)2![]() 2PbO + 4NO2 + O2
2PbO + 4NO2 + O2
(e) 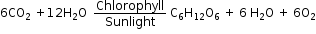
Solution D. 4
- Lead nitrate decomposes on heating, leaving a yellow residue of lead monoxide, and brown nitrogen dioxide and colourless oxygen gases are evolved.
- Due to thermal decomposition, silver chloride breaks down into silver and chloride.
- Hydrogen peroxide breaks down to form water and oxygen gas along with heat energy.
- When hydrogen sulphide is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained, and sulphuric acid so formed remains in the solution.
- A white insoluble precipitate of barium sulphate is formed.
- Quick lime reacts vigorously with water to produce slaked lime, i.e. calcium hydroxide.
- When sodium chloride is added to the silver nitrate solution, a white curdy precipitate of silver chloride is formed.
Solution D. 5
(i) Mixing (close contact): In some cases, a chemical reaction occurs when two substances are mixed in their solid states.
Example 1: Iodine and phosphorus react explosively when brought into close contact.
Example 2: Lead nitrate (white) and potassium iodide (white) react to make lead iodide (yellow).
Pb(NO3)2(s) + 2KI (s) → 2KNO3 (s) + PbI2(s)
(ii) Solution: In some cases, a chemical reaction occurs when substances are mixed in either in molten or aqueous state.
Example 1: Oxalic acid crystals and sodium carbonate react in water solution only.
Example 2: Sodium chloride and silver nitrate also react in a solution state nto form the precipoitate of silver chloride and sodium nitrate.
NaCl (aq) + AgNO3 (aq) → 2AgCl↓ + NaNO3 (aq)
White ppt.
(iii) Heat: Some chemical reactions occur only on heating.
Example 1: Copper carbonate decomposes on heating (Δ is symbol for heating) into copper oxide and carbon dioxide.
CuCO3 (s) ![]() CuO (s) + CO2 (g)
CuO (s) + CO2 (g)
Example 2: Lead nitrate decomposes on heating leaving a yellow residue of lead monoxide, brown gas nitrogen dioxide and colourless gas oxygen.
2Pb(NO3)2![]() 2PbO + 4NO2 + O2
2PbO + 4NO2 + O2
(iv) Light: Some chemical reactions take place by the action of light. These are called photochemical reactions or photolysis. Molecules of the reactants absorb light energy to get activated, and then react rapidly.
For example: Plants form glucose from carbon dioxide and water in the presence of light.
6CO2 + 12H2O ![]() C6H12O6 + 6O2 + 6H2O
C6H12O6 + 6O2 + 6H2O
Glucose
(v) Electricity: Certain chemical reactions, like the decomposition of certain compounds, occur only when electricity is passed through the reactants. Reactions that are caused or accompanied by the passage of an electric current are known as electrochemical reactions.
For example: Electrolysis of acidulated water occurs only in the presence of electricity, to give hydrogen and oxygen.
2H2O ![]() 2H2 + O2
2H2 + O2
(vi) Pressure: Some chemical reactions take place only when the involved substances are subjected to high pressure.
For example: Mercuric chloride and potassium iodide when crushed together in a mortar, give a scarlet-coloured substance called mercuric iodide.
(vii) Catalyst: Soe chemical reactions need a catalyst to accelerate or decelerate the rate at which they occur. The catalysts themselves do not take part in the reaction and so remain unchanged.
For example: Potassium chlorate decomposes oly at 700 °C and even then the rate of release of oxygen is very slow. But when potassium chlorate is heated in the presence of manganese dioxide, decomposition begins at a much lower temperature, 300 °C, and manganese dioxide remains unaffected. Thus, in this reaction, manganese dioxide acts as a catalyst.
2KClO3![]() 2KCl + 3O2
2KCl + 3O2
(a)Positive catalyst: When a catalyst accelerates the rate of a reaction, it is known as a positive catalyst.
For example: the rate of decomposition of hydrogen peroxide increases in the presence of manganese dioxide.
2H2O2![]() 2H2O + O2
2H2O + O2
(b) Negative catalyst: A catalyst employed to retard the rate of a reaction is known as a negative catalyst or inhibitor.
For example: Phosphoric acid retards the rate of decomposition of hydrogen peroxide.
Solution D. 6
(a) ![]() (Decomposition)
(Decomposition)
(b) ![]() (Single Displacement)
(Single Displacement)
(c) ![]() (Single displacement)
(Single displacement)
(d) ![]() (Decomposition)
(Decomposition)
(e) ![]() (Single displacement)
(Single displacement)
(f) ![]() (Single displacement)
(Single displacement)
(g) ![]() (Decomposition)
(Decomposition)
(h) ![]() (Double
decomposition or Double displacement)
(Double
decomposition or Double displacement)
(i) ![]() (Combination or Synthesis)
(Combination or Synthesis)
(j) ![]() (Combination or Synthesis)
(Combination or Synthesis)
Solution D. 7
Double decomposition reactions are of two types:
(a) Precipitation reactions
(b) Neutralisation reactions
(a) Precipitation reaction: A chemical reaction in which two compounds in their aqueous state react to form an insoluble salt (a precipitate) as one of the products is known as a precipitation reaction.
For example:
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
White ppt.
CuSO4(aq) + H2S(g) → CuS(s) + H2SO4(aq)
Black ppt.
Precipitation is the formation of a solid (insoluble product), either:
• When two solutions are mixed, or
• When a gas is bubbled into a solution.
Experiments: To show double decomposition reactions.
1. Take a solution of silver nitrate in a test tube ad add dilute hydrochloric acid or a solution of sodium chloride. A white, curdy precipitate is formed.
2. AgNO3 + HCl → AgCl↓ + HNO3
3. AgNO3 + NaCl → AgCl↓ + NaNO3
Note: Double decomposition reactions may also occur with evolution of a gas.
Example: On adding dilute sulphuric acid or dilute hydrochloric acid to metal sulphide we get metal sulphate or meta; chloride and an offensive (rotten egg) smelling gas H2S is evolved.
FeS(s) + H2SO4(aq) → FeSO4(aq) + H2S↑
ZnS(s) + 2HCl(aq) → ZnCl2(aq) + H2S↑
(b) Neutralisation: The reaction between an acid and a base that forms salt and water only is referred to as a reaction of neutralization.
The reaction takes place because the hydrogen ion (H+) from the acid combines with the hydroxyl ion (OH-) from the baser to form water.
NaOH + HCl → NaCl + H2O
Na+ OH⁻ + H+Cl⁻ → Na+Cl⁻ + H+OH⁻
Ionic form
Cancelling the common ions, Na+ and Cl, the only change is the combination of H+ and OH ions to form un-ionised water,
i.e., H+ + OH⁻ → H2O
In neutralization reaction, a soluble base (alkali) or an insoluble base reacts with an acid to form salt and water.
Neutralisation of soluble base (alkali) with an acid.
KOH + HNO3 → KNO3 + H2O
(Alkali) (Acid) (Salt)
Neutralisation of an insoluble base with an acid
PbO + 2HNO3 → Pb(NO3)2 + H2O
(Base) (Acid) (Salt)
Solution D. 8
Thermal decomposition:
A decomposition reaction that is brought about by heat without any recombination on cooling is known as thermal decomposition.
Example:
(a) Take some lead nitrate crydtals in a test tube and heat them. The crystals first melt and, on further heating, give out both nitrogen dioxide, a reddish brown gas, and oxygen. A yellow solid (lead monoxide) is left behind in the test tube.
2Pb(NO3)2 → 2PbO + 4NO2↑ + O2↑
(b) Put some zinc carbonate in a test tube fitted with a cork and a bent glass tube. On heating, carbon dioxide is given out, which will turn lime water milky. The residue, i.e., zinc oxide, is yellow when hot, but it turns white on cooling.
ZnCO3 → ZnO + CO2↑
Thermal dissociation:
A reversible decomposition reaction brought about only by heat is thermal dissociation.
Example:
(a) Heat some solid ammonium chloride in a test tube. Two colourless gases, ammonia and hydrogen chloride, are produced. As these gases move up to the upper part of the test tube which is cooler, they combine to form ammonium chloride, which appears as a white sublimate on the upper cooler side of the test tube.
NH4Cl ⇌ NH3↑ + HCl↑
(b) On heatring, nitrogen tetraoxide changes to nitrogen dioxide, a reddish brown gas. On cooling, nitrogen dioxide changes into the original compound, nitrogen tetraoxide.
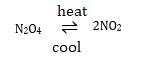
The difference between thermal decomposition and thermal dissociation reactions is that, thermal dissociation is reversible while thermal decomposition is irreversible reaction.
Solution D. 9
Metal X is more reactive than lead since, metal X displaces lead from its salt compound i.e., lead nitrate. Lead get deposited and aqueous solution of nitrate salt solution of metal X is formed.
This is displacement type of reaction.
For example:
When zinc strip is kept in aqueous solution of silver nitrate, zinc displaces sikver from its salt to form aqueous solution of silver nitrate and silver metal get deposited.
AgNO3 (aq) + Zn (s) → Zn(NO3)2 (aq) + Ag↓
Solution D. 10
The carbohydrates in rice, potatoes and bread are broken down to form glucose.
The combustion of glucose with oxygen in the cells of the body provides energy:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
This reaction is respiration. Due to evolution of energy, respiration is an exothermic type of reaction.
Solution D. 11
Digestion of food by our body is an example of a decomposition reaction.
The starch present in the food we eat decomposes into glucose and sugar. Proteins undergo decomposition to from amino acids. Proteins undergo decomposition to form amino acids. Fats and oils are decomposed to fatty acids and finally oxidized by respiration into carbon dioxide and water.
![]()
Solution E. 1
(a) Metal hydroxide on heating forms metal oxide and water vapour.
Ca(OH)2 → CaO + H2O
(b) Metal hydroxide on heating forms metal, oxygen and water vapours.
4AgOH → 4Ag + O2 + 2H2O
(c) Metal nitrate decomposes to give two products only.
2KNO3 → 2KNO2 + O2
(d) Metal nitrate on heating forms metal oxide, nitrogen dioxide and oxygen.
2Zn(NO3)2 → 2ZnO + 4NO2 + O2
(e) Metal nitrate on heating forms metal, nitrogen dioxide and oxygen.
![]()
(f) Metal carbonate which is stable to heat.
K2CO3 and Na2CO3 are stable to heat.
(g) Metal carbonate which forms metal oxide and carbon dioxide on heating.
CuCO3 → CuO + CO2
(h) The heating effect on bivalent metal hydrogen carbonate.
![]()
Solution E. 2
(a) The balanced equation is as follows:
2KI + Cl2 → 2KCl + I2
(b) This is the displacement type of reaction.
(c) Chlorine is able to displace iodine from its compound potassium iodide, hence chlorine is more reactive than iodine.
(d) There will be no reaction if KCl reacts with iodine as iodine is unable to displace chlorine from its compound due to its less reactive nature of iodine than chlorine.