CBSE Class 12-science - Three Dimensional Packing Videos
Close packing in 3 dimensions
Close packing in 3Dimensions (a) Hexagonal close packing (b) Cubic close packing
- what is solid
- The unit cell of Na is bcc and its density is 0.97. What is the radius of a sodium atom if the molar mass of Na is 23?
-
explain please

-
pls solve sir
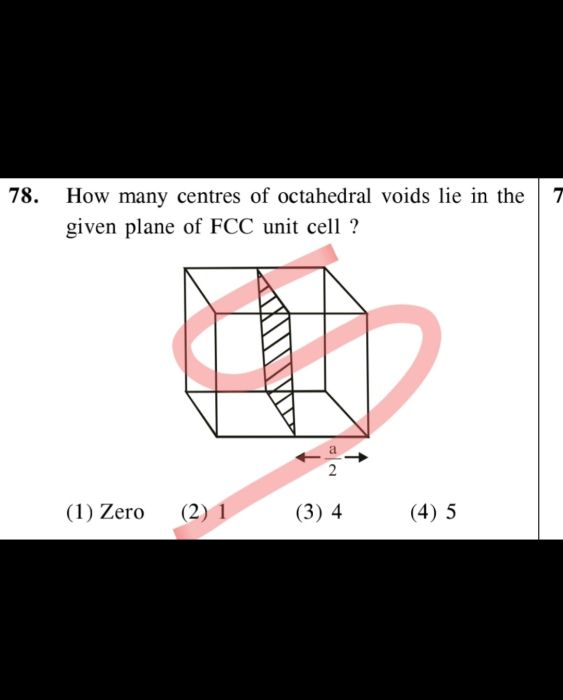
- How many types of 3dimensions are there in a lattice please define.
- sir in types of unit cell we studied it can be of 4 types i.e primitive, face centred, body centred and end centred, but while studing 3D close packing it was told that it can be of 3 types i,e. simple(primitive) cubic unit cell, hcp and ccp. but i am not able to understand that hcp is a type of unit cell or it is one of the 7 crystal system or somthing else?and if it is type of unit cell then why it is not taught under the topic types of unit cell. and if it is a crystal system then what about the rest 6 crystal system, then why rest 6 crystal system does not comes under 3 dimensional close packing?
- In a cubic lattice A atoms having FCC arrangement B atoms are at body centers and C atoms are at alternate edge centers . The formula of the unit cell will be ;
- The number of c4 and c3- axes in a cubic crystal lattice are x and y respectively the value of x+y= a] 2 b] 7
- Hexagonal close packing and cubic close packing
- Analysis shows that nickel oxide has the formula ni 0.98 o1.00. What fractions of nickel exist as ni 2+ and ni 3+ ions?

